Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation
- PMID: 15664921
- PMCID: PMC547028
- DOI: 10.1128/IAI.73.2.820-827.2005
Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation
Abstract
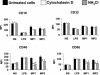
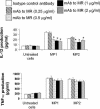
Our previous data show that mannoproteins (MPs) from Cryptococcus neoformans are able to induce protective responses against both C. neoformans and Candida albicans. Here we provide evidence that MPs foster maturation and activation of human dendritic cells (DCs). Maturation was evaluated by the ability of MPs to facilitate expression of costimulatory molecules such as CD40, CD86, CD83, and major histocompatibility complex classes I and II and to inhibit receptors such as CD14, CD16, and CD32. Activation of DCs was measured by the capacity of MPs to promote interleukin-12 and tumor necrosis factor alpha secretion. DC-induced maturation and interleukin-12 induction are largely mediated by engagement of mannose receptors and presume MP internalization and degradation. DC activation leads to IkappaBalpha phosphorylation, which is necessary for nuclear factor kappaB transmigration into the nucleus. MP-loaded DCs are efficient stimulators of T cells and show a remarkable capacity to promote CD4 and CD8 proliferation. In conclusion, we have evidenced a novel regulatory role of MPs that promotes their candidacy as a vaccine against fungi.
Figures


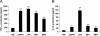

References
-
- Bowie, A., and L. A. O'Neill. 2000. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 59:13-23. - PubMed
-
- Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans, 1st ed. ASM Press, Washington, D.C.
-
- Chaka, W., A. F. Verheul, V. V. Vaishnav, R. Cherniak, J. Scharringa, J. Verhoef, H. Snippe, and A. I. Hoepelman. 1997. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J. Immunol. 159:2979-2985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

