Solubility and bioactivity of the Pseudomonas quinolone signal are increased by a Pseudomonas aeruginosa-produced surfactant
- PMID: 15664929
- PMCID: PMC547021
- DOI: 10.1128/IAI.73.2.878-882.2005
Solubility and bioactivity of the Pseudomonas quinolone signal are increased by a Pseudomonas aeruginosa-produced surfactant
Abstract
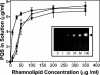
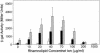
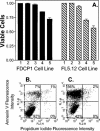
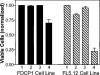
Pseudomonas aeruginosa is a gram-negative bacterium that causes serious infections in immunocompromised individuals and cystic fibrosis patients. This opportunistic pathogen controls many of its virulence factors and cellular functions through the activity of three cell-to-cell signals, N-(3-oxododecanoyl)-L-homoserine lactone, N-butyryl-L-homoserine lactone, and the Pseudomonas quinolone signal (PQS). The activity of these signals is dependent upon their ability to dissolve in and freely diffuse through the aqueous solution in which P. aeruginosa happens to reside. Despite this, our data indicated that PQS was relatively insoluble in aqueous solutions, which led us to postulate that P. aeruginosa could be producing a PQS-solubilizing factor. In this report, we show that the P. aeruginosa-produced biosurfactant rhamnolipid greatly enhances the solubility of PQS in aqueous solutions. The enhanced solubility of PQS led to an increase in PQS bioactivity, as measured by both a gene induction assay and an apoptosis assay. This is the first demonstration of the importance of a bacterial surfactant in the solubilization and bioactivity of a cell-to-cell signal.
Figures

References
-
- Bromberg, K. D., A. B. Burgin, and N. Osheroff. 2003. Quinolone action against human topoisomerase IIalpha: stimulation of enzyme-mediated double-stranded DNA cleavage. Biochemistry 42:3393-3398. - PubMed
-
- Collier, D. N., L. Anderson, S. L. McKnight, T. L. Noah, M. Knowles, R. Boucher, U. Schwab, P. Gilligan, and E. C. Pesci. 2002. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol. Lett. 215:41-46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

