Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes
- PMID: 15664931
- PMCID: PMC546953
- DOI: 10.1128/IAI.73.2.894-904.2005
Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes
Abstract
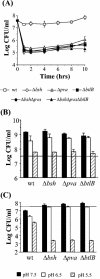
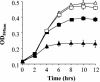
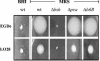
Listeria monocytogenes must resist the deleterious actions of bile in order to infect and subsequently colonize the human gastrointestinal tract. The molecular mechanisms used by the bacterium to resist bile and the influence of bile on pathogenesis are as yet largely unexplored. This study describes the analysis of three genes--bsh, pva, and btlB--previously annotated as bile-associated loci in the sequenced L. monocytogenes EGDe genome (lmo2067, lmo0446, and lmo0754, respectively). Analysis of deletion mutants revealed a role for all three genes in resisting the acute toxicity of bile and bile salts, particularly glycoconjugated bile salts at low pH. Mutants were unaffected in the other stress responses examined (acid, salt, and detergents). Bile hydrolysis assays demonstrate that L. monocytogenes possesses only one bile salt hydrolase gene, namely, bsh. Transcriptional analyses and activity assays revealed that, although it is regulated by both PrfA and sigma(B), the latter appears to play the greater role in modulating bsh expression. In addition to being incapable of bile hydrolysis, a sigB mutant was shown to be exquisitely sensitive to bile salts. Furthermore, increased expression of sigB was detected under anaerobic conditions and during murine infection. A gene previously annotated as a possible penicillin V amidase (pva) or bile salt hydrolase was shown to be required for resistance to penicillin V but not penicillin G but did not demonstrate a role in bile hydrolysis. Finally, animal (murine) studies revealed an important role for both bsh and btlB in the intestinal persistence of L. monocytogenes.
Figures




References
-
- Allerberger, F., B. Langer, O. Hirsch, M. P. Dierich, and H. P. Seeliger. 1989. Listeria monocytogenes cholecystitis. Z. Gastroenterol. 27:145-147. - PubMed
-
- Aries, V., and M. J. Hill. 1970. Degradation of steroids by intestinal bacteria. I. Deconjugation of bile salts. Biochim. Biophys. Acta 202:526-534. - PubMed
-
- Begley, M. 2003. Physiology and genetics of bile tolerance in Listeria monocytogenes. Ph.D. thesis. National University of Ireland, University College Cork, Cork, Ireland.
-
- Begley, M., C. G. M. Gahan, and C. Hill. The interaction between bacteria and bile. FEMS Microbiol. Rev., in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

