Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin
- PMID: 15664949
- PMCID: PMC547010
- DOI: 10.1128/IAI.73.2.1052-1060.2005
Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin
Abstract
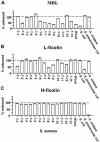
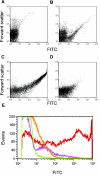
Mannan-binding lectin (MBL), L-ficolin, and H-ficolin are pattern recognition molecules of the innate immune system. We investigated their ability to bind to different serotypes and noncapsulated variants of two gram-positive bacterial species, Streptococcus pneumoniae and Staphylococcus aureus. MBL did not bind to capsulated S. aureus or capsulated S. pneumoniae but did bind to a noncapsulated S. aureus variant (Wood). L-ficolin bound to some capsulated S. aureus serotypes (serotypes 1, 8, 9, 11, and 12) and capsulated S. pneumoniae serotypes (11A, 11D, and 11F) but not to noncapsulated strains. H-ficolin did not bind to any of the S. pneumoniae and S. aureus serotypes included in this study but did bind to one strain of Aerococcus viridans. The concentrations of the three proteins in 97 plasma samples were estimated. The median concentrations were 0.8 mug per ml for MBL, 3.3 mug per ml for L-ficolin, and 18.4 mug per ml for H-ficolin.
Figures
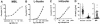



References
-
- Akaiwa, M., Y. Yae, R. Sugimoto, S. O. Suzuki, T. Iwaki, K. Izuhara, and N. Hamasaki. 1999. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J. Histochem. Cytochem. 47:777-786. - PubMed
-
- Dagnaes-Hansen, F., M. Kilian, and K. Fuursted. 2004. Septicaemia associated with an Aerococcus viridans infection in immunodeficient mice. Lab. Anim. 38:321-325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

