Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54
- PMID: 15665093
- PMCID: PMC545088
- DOI: 10.1073/pnas.0409330102
Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54
Abstract
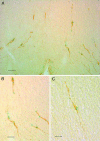
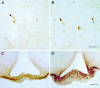
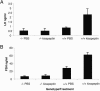
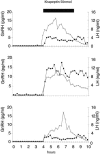
We have recently described a molecular gatekeeper of the hypothalamic-pituitary-gonadal axis with the observation that G protein-coupled receptor 54 (GPR54) is required in mice and men for the pubertal onset of pulsatile luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion to occur. In the present study, we investigate the possible central mode of action of GPR54 and kisspeptin ligand. First, we show that GPR54 transcripts are colocalized with gonadotropin-releasing hormone (GnRH) neurons in the mouse hypothalamus, suggesting that kisspeptin, the GPR54 ligand, may act directly on these neurons. Next, we show that GnRH neurons seem anatomically normal in gpr54-/- mice, and that they show projections to the median eminence, which demonstrates that the hypogonadism in gpr54-/- mice is not due to an abnormal migration of GnRH neurons (as occurs with KAL1 mutations), but that it is more likely due to a lack of GnRH release or absence of GnRH neuron stimulation. We also show that levels of kisspeptin injected i.p., which stimulate robust LH and FSH release in wild-type mice, have no effect in gpr54-/- mice, and therefore that kisspeptin acts directly and uniquely by means of GPR54 signaling for this function. Finally, we demonstrate by direct measurement, that the central administration of kisspeptin intracerebroventricularly in sheep produces a dramatic release of GnRH into the cerebrospinal fluid, with a parallel rise in serum LH, demonstrating that a key action of kisspeptin on the hypothalamo-pituitary-gonadal axis occurs directly at the level of GnRH release. The localization and GnRH release effects of kisspeptin thus define GPR54 as a major control point in the reproductive axis and suggest kisspeptin to be a neurohormonal effector.
Figures

Similar articles
-
Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat.Neuroendocrinology. 2004;80(4):264-72. doi: 10.1159/000083140. Epub 2005 Jan 5. Neuroendocrinology. 2004. PMID: 15665556
-
Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice.Endocrinology. 2012 Jan;153(1):316-28. doi: 10.1210/en.2011-1260. Epub 2011 Nov 8. Endocrinology. 2012. PMID: 22067321
-
Kisspeptin-gpr54 signaling at the GnRH neuron is necessary for negative feedback regulation of luteinizing hormone secretion in female mice.Neuroendocrinology. 2014;100(2-3):191-7. doi: 10.1159/000368608. Epub 2014 Dec 18. Neuroendocrinology. 2014. PMID: 25301053
-
KiSS-1 and GPR54 as new players in gonadotropin regulation and puberty.Endocrine. 2005 Apr;26(3):277-84. doi: 10.1385/ENDO:26:3:277. Endocrine. 2005. PMID: 16034182 Review.
-
Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion.Endocrinology. 2006 Mar;147(3):1154-8. doi: 10.1210/en.2005-1282. Epub 2005 Dec 22. Endocrinology. 2006. PMID: 16373418 Review.
Cited by
-
Kisspeptin neurons from mice to men: similarities and differences.Endocrinology. 2012 Nov;153(11):5105-18. doi: 10.1210/en.2012-1550. Epub 2012 Sep 18. Endocrinology. 2012. PMID: 22989628 Free PMC article. Review.
-
Cancer susceptibility and reproductive trade-offs: a model of the evolution of cancer defences.Philos Trans R Soc Lond B Biol Sci. 2015 Jul 19;370(1673):20140220. doi: 10.1098/rstb.2014.0220. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26056364 Free PMC article.
-
Interactions between Two Different G Protein-Coupled Receptors in Reproductive Hormone-Producing Cells: The Role of PACAP and Its Receptor PAC1R.Int J Mol Sci. 2016 Sep 26;17(10):1635. doi: 10.3390/ijms17101635. Int J Mol Sci. 2016. PMID: 27681724 Free PMC article. Review.
-
Evolutionary Insights into the Steroid Sensitive kiss1 and kiss2 Neurons in the Vertebrate Brain.Front Endocrinol (Lausanne). 2012 Feb 22;3:28. doi: 10.3389/fendo.2012.00028. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22654859 Free PMC article.
-
Nutrition, Growth, and Age at Puberty in Heifers.Animals (Basel). 2024 Sep 27;14(19):2801. doi: 10.3390/ani14192801. Animals (Basel). 2024. PMID: 39409750 Free PMC article. Review.
References
-
- Ebling, F. J. & Cronin, A. S. (2000) NeuroReport 11, R23–R33. - PubMed
-
- Franco, B., Guioli, S., Pragliola, A., Incerti, B., Bardoni, B., Tonlorenzi, R., Carrozzo, R., Maestrini, E., Pieretti, M., Taillon-Miller, P., et al. (1991) Nature 353, 529–536. - PubMed
-
- Legouis, R., Hardelin, J. P., Levilliers, J., Claverie, J. M., Compain, S., Wunderle, V., Millasseau, P., Le Paslier, D., Cohen, D., Caterina, D., et al. (1991) Cell 67, 423–435. - PubMed
-
- Schwarting, G. A., Raitcheva, D., Bless, E. P., Ackerman, S. L. & Tobet, S. (2004) Eur. J. Neurosci. 19, 11–20. - PubMed
-
- Seminara, S. B., Messager, S., Chatzidaki, E. E., Thresher, R. R., Acierno, J. S., Jr., Shagoury, J. K., Bo-Abbas, Y., Kuohung, W., Schwinof, K. M., Hendrick, A. G., et al. (2003) N. Engl. J. Med. 349, 1614–1627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

