Gating of acetylcholine receptor channels: brownian motion across a broad transition state
- PMID: 15665102
- PMCID: PMC547815
- DOI: 10.1073/pnas.0406787102
Gating of acetylcholine receptor channels: brownian motion across a broad transition state
Abstract
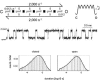
Acetylcholine receptor channels (AChRs) are proteins that switch between stable "closed" and "open" conformations. In patch clamp recordings, diliganded AChR gating appears to be a simple, two-state reaction. However, mutagenesis studies indicate that during gating dozens of residues across the protein move asynchronously and are organized into rigid body gating domains ("blocks"). Moreover, there is an upper limit to the apparent channel opening rate constant. These observations suggest that the gating reaction has a broad, corrugated transition state region, with the maximum opening rate reflecting, in part, the mean first-passage time across this ensemble. Simulations reveal that a flat, isotropic energy profile for the transition state can account for many of the essential features of AChR gating. With this mechanism, concerted, local structural transitions that occur on the broad transition state ensemble give rise to fractional measures of reaction progress (Phi values) determined by rate-equilibrium free energy relationship analysis. The results suggest that the coarse-grained AChR gating conformational change propagates through the protein with dynamics that are governed by the Brownian motion of individual gating blocks.
Figures
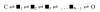
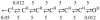
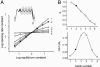
References
-
- Doyle, D. A., Morais Cabral, J., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T. & MacKinnon, R. (1998) Science 280, 69-77. - PubMed
-
- Jiang, Y., Lee, A., Chen, J., Cadene, M., Chait, B. T. & MacKinnon, R. (2002) Nature 417, 523-526. - PubMed
-
- Miyazawa, A., Fujiyoshi, Y. & Unwin, N. (2003) Nature 424, 949-955. - PubMed
-
- Liu, Y. S., Sompornpisut, P. & Perozo, E. (2001) Nat. Struct. Biol. 8, 883-887. - PubMed
-
- Karlin, A. (2002) Nat. Rev. Neurosci. 3, 102-114. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

