Attenuation of cardiac remodeling after myocardial infarction by muscle LIM protein-calcineurin signaling at the sarcomeric Z-disc
- PMID: 15665106
- PMCID: PMC547821
- DOI: 10.1073/pnas.0405488102
Attenuation of cardiac remodeling after myocardial infarction by muscle LIM protein-calcineurin signaling at the sarcomeric Z-disc
Abstract
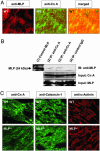
Adverse left ventricular (LV) remodeling after myocardial infarction (MI) is a major cause for heart failure. Molecular modifiers of the remodeling process remain poorly defined. Patients with heart failure after MI have reduced LV expression levels of muscle LIM protein (MLP), a component of the sarcomeric Z-disk that is involved in the integration of stress signals in cardiomyocytes. By using heterozygous MLP mutant (MLP+/-) mice, we explored the role of MLP in post-MI remodeling. LV dimensions and function were similar in sham-operated WT and MLP+/- mice. After MI, however, MLP+/- mice displayed more pronounced LV dilatation and systolic dysfunction and decreased survival compared with WT mice, indicating that reduced MLP levels predispose to adverse LV remodeling. LV dilatation in MLP+/- mice was associated with reduced thickening but enhanced elongation of cardiomyocytes. Activation of the stress-responsive, prohypertrophic calcineurin-nuclear factor of activated T-cells (NFAT) signaling pathway was reduced in MLP+/- mice after MI, as shown by a blunted transcriptional activation of NFAT in cardiomyocytes isolated from MLP+/-/NFAT-luciferase reporter gene transgenic mice. Calcineurin was colocalized with MLP at the Z-disk in WT mice but was displaced from the Z-disk in MLP+/- mice, indicating that MLP is essential for calcineurin anchorage to the Z-disk. In vitro assays in cardiomyocytes with down-regulated MLP confirmed that MLP is required for stress-induced calcineurin-NFAT activation. Our study reveals a link between the stress sensor MLP and the calcineurin-NFAT pathway at the sarcomeric Z-disk in cardiomyocytes and indicates that reduced MLP-calcineurin signaling predisposes to adverse remodeling after MI.
Figures




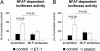
References
-
- Gheorghiade, M. & Bonow, R. O. (1998) Circulation 97, 282–289. - PubMed
-
- Cohn, J. N., Ferrari, R. & Sharpe, N. (2000) J. Am. Coll. Cardiol. 35, 569–582. - PubMed
-
- Mann, D. L. (1999) Circulation 100, 999–1008. - PubMed
-
- Force, T., Michael, A., Kilter, H. & Haq, S. (2002) J. Card. Fail. 8, S351–S358. - PubMed
-
- Zimmer, H. G., Gerdes, A. M., Lortet, S. & Mall, G. (1990) J. Mol. Cell. Cardiol. 22, 1231–1243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

