Probing the viscoelastic behavior of cultured airway smooth muscle cells with atomic force microscopy: stiffening induced by contractile agonist
- PMID: 15665124
- PMCID: PMC1305393
- DOI: 10.1529/biophysj.104.046649
Probing the viscoelastic behavior of cultured airway smooth muscle cells with atomic force microscopy: stiffening induced by contractile agonist
Abstract
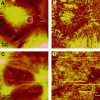
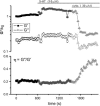
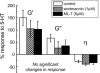
Complex rheology of airway smooth muscle cells and its dynamic response during contractile stimulation involves many molecular processes, foremost of which are actomyosin cross-bridge cycling and actin polymerization. With an atomic force microscope, we tracked the spatial and temporal variations of the viscoelastic properties of cultured airway smooth muscle cells. Elasticity mapping identified stiff structural elements of the cytoskeletal network. Using a precisely positioned microscale probe, picoNewton forces and nanometer level indentation modulations were applied to cell surfaces at frequencies ranging from 0.5 to 100 Hz. The resulting elastic storage modulus (G') and dissipative modulus (G'') increased dramatically, with hysteresivity (eta = G''/G') showing a definitive decrease after stimulation with the contractile agonist 5-hydroxytryptamine. Frequency-dependent assays showed weak power-law structural damping behavior and universal scaling in support of the soft-glassy material description of cellular biophysics. Additionally, a high-frequency component of the loss modulus (attributed to cellular Newtonian viscosity) increased fourfold during the contractile process. The complex shear modulus showed a strong sensitivity to the degree of actin polymerization. Inhibitors of myosin light chain kinase activity had little effect on the stiffening response to contractile stimulation. Thus, our measurements appear to be particularly well suited for characterization of dynamic actin rheology during airway smooth muscle contraction.
Figures



Similar articles
-
Microrheology of human lung epithelial cells measured by atomic force microscopy.Biophys J. 2003 Mar;84(3):2071-9. doi: 10.1016/S0006-3495(03)75014-0. Biophys J. 2003. PMID: 12609908 Free PMC article.
-
Stiffness changes in cultured airway smooth muscle cells.Am J Physiol Cell Physiol. 2002 Sep;283(3):C792-801. doi: 10.1152/ajpcell.00425.2001. Am J Physiol Cell Physiol. 2002. PMID: 12176736
-
Rat airway smooth muscle cell during actin modulation: rheology and glassy dynamics.Am J Physiol Cell Physiol. 2005 Dec;289(6):C1388-95. doi: 10.1152/ajpcell.00060.2005. Epub 2005 Aug 24. Am J Physiol Cell Physiol. 2005. PMID: 16120653
-
Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung.Respir Physiol Neurobiol. 2003 Sep 16;137(2-3):151-68. doi: 10.1016/s1569-9048(03)00144-7. Respir Physiol Neurobiol. 2003. PMID: 14516723 Review.
-
The contractile apparatus and mechanical properties of airway smooth muscle.Eur Respir J. 2000 Mar;15(3):600-16. doi: 10.1034/j.1399-3003.2000.15.29.x. Eur Respir J. 2000. PMID: 10759460 Review.
Cited by
-
Scaling-law mechanical marker for liver fibrosis diagnosis and drug screening through machine learning.Front Bioeng Biotechnol. 2024 Jul 16;12:1404508. doi: 10.3389/fbioe.2024.1404508. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39081332 Free PMC article.
-
Fast fluorescence laser tracking microrheometry. I: instrument development.Biophys J. 2008 Feb 15;94(4):1459-69. doi: 10.1529/biophysj.106.098111. Epub 2007 Oct 26. Biophys J. 2008. PMID: 17965137 Free PMC article.
-
Frequency-dependent transition in power-law rheological behavior of living cells.Sci Adv. 2022 May 6;8(18):eabn6093. doi: 10.1126/sciadv.abn6093. Epub 2022 May 6. Sci Adv. 2022. PMID: 35522746 Free PMC article.
-
Cell stiffening in response to external stress is correlated to actin recruitment.Biophys J. 2008 Apr 1;94(7):2906-13. doi: 10.1529/biophysj.107.118265. Epub 2008 Jan 4. Biophys J. 2008. PMID: 18178644 Free PMC article.
-
Dynamic nanoindentation by instrumented nanoindentation and force microscopy: a comparative review.Beilstein J Nanotechnol. 2013 Nov 29;4:815-33. doi: 10.3762/bjnano.4.93. Beilstein J Nanotechnol. 2013. PMID: 24367751 Free PMC article. Review.
References
-
- Alcaraz, J., L. Buscemi, M. Puig-De-Morales, J. Colchero, A. Baro, and D. Navajas. 2002. Correction of microrheological measurements of soft samples with atomic force microscopy for the hydrodynamic drag on the cantilever. Langmuir. 18:716–721.
-
- An, S. S., R. E. Laudadio, J. Lai, R. A. Rogers, and J. J. Fredberg. 2002. Stiffness changes in cultured airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 283:C792–C801. - PubMed
-
- Ashton, F. T., A. V. Somlyo, and A. P. Somlyo. 1975. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J. Mol. Biol. 98:17–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

