Protein self-association induced by macromolecular crowding: a quantitative analysis by magnetic relaxation dispersion
- PMID: 15665132
- PMCID: PMC1305380
- DOI: 10.1529/biophysj.104.055871
Protein self-association induced by macromolecular crowding: a quantitative analysis by magnetic relaxation dispersion
Abstract
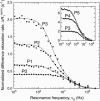
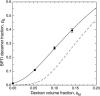
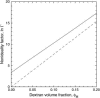
In the presence of high concentrations of inert macromolecules, the self-association of proteins is strongly enhanced through an entropic, excluded-volume effect variously called macromolecular crowding or depletion attraction. Despite the predicted large magnitude of this universal effect and its far-reaching biological implications, few experimental studies of macromolecular crowding have been reported. Here, we introduce a powerful new technique, fast field-cycling magnetic relaxation dispersion, for investigating crowding effects on protein self-association equilibria. By recording the solvent proton spin relaxation rate over a wide range of magnetic field strengths, we determine the populations of coexisting monomers and decamers of bovine pancreatic trypsin inhibitor in the presence of dextran up to a macromolecular volume fraction of 27%. Already at a dextran volume fraction of 14%, we find a 30-fold increase of the decamer population and 510(5)-fold increase of the association constant. The analysis of these results, in terms of a statistical-mechanical model that incorporates polymer flexibility as well as the excluded volume of the protein, shows that the dramatic enhancement of bovine pancreatic trypsin inhibitor self-association can be quantitatively rationalized in terms of hard repulsive interactions.
Figures



Similar articles
-
Protein self-association in solution: the bovine pancreatic trypsin inhibitor decamer.Biophys J. 2003 Jun;84(6):3941-58. doi: 10.1016/S0006-3495(03)75122-4. Biophys J. 2003. PMID: 12770900 Free PMC article.
-
Effects of macromolecular crowding on biochemical reaction equilibria: a molecular thermodynamic perspective.Biophys J. 2007 Sep 1;93(5):1464-73. doi: 10.1529/biophysj.107.104646. Epub 2007 May 18. Biophys J. 2007. PMID: 17513384 Free PMC article.
-
Attractive protein-polymer interactions markedly alter the effect of macromolecular crowding on protein association equilibria.Biophys J. 2010 Aug 4;99(3):914-23. doi: 10.1016/j.bpj.2010.05.013. Biophys J. 2010. PMID: 20682270 Free PMC article.
-
Macromolecular Crowding Is More than Hard-Core Repulsions.Annu Rev Biophys. 2022 May 9;51:267-300. doi: 10.1146/annurev-biophys-091321-071829. Epub 2022 Mar 3. Annu Rev Biophys. 2022. PMID: 35239418 Review.
-
Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes.Chem Rev. 2009 Sep;109(9):4108-39. doi: 10.1021/cr900033p. Chem Rev. 2009. PMID: 19522502 Free PMC article. Review. No abstract available.
Cited by
-
Formation of protein complexes in crowded environments--from in vitro to in vivo.FEBS Lett. 2013 Apr 17;587(8):1046-52. doi: 10.1016/j.febslet.2013.01.007. Epub 2013 Jan 18. FEBS Lett. 2013. PMID: 23337873 Free PMC article. Review.
-
Serum albumin prevents protein aggregation and amyloid formation and retains chaperone-like activity in the presence of physiological ligands.J Biol Chem. 2012 Jun 15;287(25):21530-40. doi: 10.1074/jbc.M112.372961. Epub 2012 May 1. J Biol Chem. 2012. PMID: 22549788 Free PMC article.
-
Macromolecular crowding modulates folding mechanism of alpha/beta protein apoflavodoxin.Biophys J. 2009 Jan;96(2):671-80. doi: 10.1016/j.bpj.2008.10.014. Biophys J. 2009. PMID: 19167312 Free PMC article.
-
Macromolecular Crowding In Vitro, In Vivo, and In Between.Trends Biochem Sci. 2016 Nov;41(11):970-981. doi: 10.1016/j.tibs.2016.08.013. Epub 2016 Sep 23. Trends Biochem Sci. 2016. PMID: 27669651 Free PMC article. Review.
-
Influence of crowded cellular environments on protein folding, binding, and oligomerization: biological consequences and potentials of atomistic modeling.FEBS Lett. 2013 Apr 17;587(8):1053-61. doi: 10.1016/j.febslet.2013.01.064. Epub 2013 Feb 5. FEBS Lett. 2013. PMID: 23395796 Free PMC article. Review.
References
-
- Albertsson, P.-Å. 1986. Partition of Cell Particles and Macromolecules. Wiley-Interscience, New York.
-
- Al-Habori, M. 2001. Macromolecular crowding and its role as intracellular signaling of cell volume regulation. Int. J. Biochem. Cell Biol. 33:844–864. - PubMed
-
- Asakura, S., and F. Oosawa. 1954. On interaction between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 22:1255–1256.
-
- Asakura, S., and F. Oosawa. 1958. Interaction between particles suspended in solutions of macromolecules. J. Polym. Sci. [B]. 33:183–192.
-
- Atha, D. A., and K. C. Ingham. 1981. Mechanism of precipitation of proteins by polyethylene glycols. J. Biol. Chem. 256:12108–12117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

