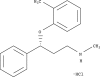
Atomoxetine: a new pharmacotherapeutic approach in the management of attention deficit/hyperactivity disorder
- PMID: 15665154
- PMCID: PMC1765270
- DOI: 10.1136/adc.2004.059386
Atomoxetine: a new pharmacotherapeutic approach in the management of attention deficit/hyperactivity disorder
Abstract
Atomoxetine is a novel, non-stimulant, highly selective noradrenaline reuptake inhibitor that has been studied for use in the treatment of attention deficit/hyperactivity disorder (ADHD). Data from clinical trials show it to be well tolerated and effective in the treatment of ADHD in children, adolescents, and adults. Improvements were seen not only in core symptoms of ADHD, but also in broader social and family functioning and self esteem. Once-daily dosing of atomoxetine has been shown to be effective in providing continuous symptom relief. Atomoxetine does not appear to have abuse potential and is associated with a benign side effect profile. The development of atomoxetine thus represents an important advance in the pharmacological management of ADHD.
Similar articles
-
[Atomoxetine for the treatment of attention-deficit/hyperactivity disorder].Fortschr Neurol Psychiatr. 2004 Oct;72(10):586-91. doi: 10.1055/s-2004-830049. Fortschr Neurol Psychiatr. 2004. PMID: 15472782 German.
-
Evolution of the treatment of attention-deficit/hyperactivity disorder in children: a review.Clin Ther. 2008 May;30(5):942-57. doi: 10.1016/j.clinthera.2008.05.006. Clin Ther. 2008. PMID: 18555941 Review.
-
[Atomoxetine safety and efficacy in children with attention deficit/hyperactivity disorder (ADHD): initial phase of 10-week treatment in a relapse prevention study with a Spanish sample].Actas Esp Psiquiatr. 2005 Jan-Feb;33(1):26-32. Actas Esp Psiquiatr. 2005. PMID: 15704028 Spanish.
-
Atomoxetine for attention deficit/hyperactivity disorder.Issues Emerg Health Technol. 2003 May;(46):1-4. Issues Emerg Health Technol. 2003. PMID: 12751480
-
Atomoxetine in the treatment of attention deficit hyperactivity disorder.Expert Rev Neurother. 2004 Jul;4(4):601-11. doi: 10.1586/14737175.4.4.601. Expert Rev Neurother. 2004. PMID: 15853579 Review.
Cited by
-
Safety and Tolerability of Lisdexamfetamine: A Retrospective Cohort Study.CNS Drugs. 2015 May;29(5):415-23. doi: 10.1007/s40263-015-0246-y. CNS Drugs. 2015. PMID: 25920467
-
Viloxazine for the Treatment of Attention Deficit Hyperactivity Disorder.Health Psychol Res. 2022 Sep 23;10(3):38360. doi: 10.52965/001c.38360. eCollection 2022. Health Psychol Res. 2022. PMID: 36168642 Free PMC article.
-
Withdrawal Dyskinesia Associated With Aripiprazole in a Child: A Case Report.Cureus. 2024 Jul 23;16(7):e65223. doi: 10.7759/cureus.65223. eCollection 2024 Jul. Cureus. 2024. PMID: 39184787 Free PMC article.
-
(1)H-Magnetic resonance spectroscopy study of stimulant medication effect on brain metabolites in French Canadian children with attention deficit hyperactivity disorder.Neuropsychiatr Dis Treat. 2014 Jan 17;10:47-54. doi: 10.2147/NDT.S52338. eCollection 2014. Neuropsychiatr Dis Treat. 2014. PMID: 24476627 Free PMC article.
-
Current Pharmacological Treatments for ADHD.Curr Top Behav Neurosci. 2022;57:19-50. doi: 10.1007/7854_2022_330. Curr Top Behav Neurosci. 2022. PMID: 35507282 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical