Reductions of Rubisco activase by antisense RNA in the C4 plant Flaveria bidentis reduces Rubisco carbamylation and leaf photosynthesis
- PMID: 15665240
- PMCID: PMC1065374
- DOI: 10.1104/pp.104.056077
Reductions of Rubisco activase by antisense RNA in the C4 plant Flaveria bidentis reduces Rubisco carbamylation and leaf photosynthesis
Abstract
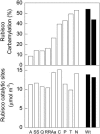
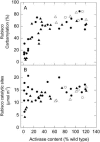
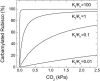
To function, the catalytic sites of Rubisco (EC 4.1.1.39) need to be activated by the reversible carbamylation of a lysine residue within the sites followed by rapid binding of magnesium. The activation of Rubisco in vivo requires the presence of the regulatory protein Rubisco activase. This enzyme is thought to aid the release of sugar phosphate inhibitors from Rubisco's catalytic sites, thereby influencing carbamylation. In C3 species, Rubisco operates in a low CO2 environment, which is suboptimal for both catalysis and carbamylation. In C4 plants, Rubisco is located in the bundle sheath cells and operates in a high CO2 atmosphere close to saturation. To explore the role of Rubisco activase in C4 photosynthesis, activase levels were reduced in Flaveria bidentis, a C4 dicot, by transformation with an antisense gene directed against the mRNA for Rubisco activase. Four primary transformants with very low activase levels were recovered. These plants and several of their segregating T1 progeny required high CO2 (>1 kPa) for growth. They had very low CO2 assimilation rates at high light and ambient CO2, and only 10% to 15% of Rubisco sites were carbamylated at both ambient and very high CO2. The amount of Rubisco was similar to that of wild-type plants. Experiments with the T1 progeny of these four primary transformants showed that CO2 assimilation rate and Rubisco carbamylation were severely reduced in plants with less than 30% of wild-type levels of activase. We conclude that activase activity is essential for the operation of the C4 photosynthetic pathway.
Figures





Similar articles
-
The effects of Rubisco activase on C4 photosynthesis and metabolism at high temperature.J Exp Bot. 2008;59(7):1789-98. doi: 10.1093/jxb/erm373. Epub 2008 Mar 28. J Exp Bot. 2008. PMID: 18375609
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase activase deficiency delays senescence of ribulose-1,5-bisphosphate carboxylase/oxygenase but progressively impairs its catalysis during tobacco leaf development.Plant Physiol. 1997 Dec;115(4):1569-80. doi: 10.1104/pp.115.4.1569. Plant Physiol. 1997. PMID: 9414564 Free PMC article.
-
Reduction of ribulose biphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose biphosphate carboxylase carbamylation and impairs photosynthesis.Plant Physiol. 1993 Aug;102(4):1119-28. doi: 10.1104/pp.102.4.1119. Plant Physiol. 1993. PMID: 8278543 Free PMC article.
-
Strategies for improving C4 photosynthesis.Curr Opin Plant Biol. 2016 Jun;31:125-34. doi: 10.1016/j.pbi.2016.04.003. Epub 2016 Apr 27. Curr Opin Plant Biol. 2016. PMID: 27127850 Review.
-
Rubisco gene expression in C4 plants.J Exp Bot. 2008;59(7):1625-34. doi: 10.1093/jxb/erm368. Epub 2008 Mar 5. J Exp Bot. 2008. PMID: 18325924 Review.
Cited by
-
Towards a dynamic photosynthesis model to guide yield improvement in C4 crops.Plant J. 2021 Jul;107(2):343-359. doi: 10.1111/tpj.15365. Epub 2021 Aug 6. Plant J. 2021. PMID: 34087011 Free PMC article.
-
Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize.Plants (Basel). 2023 Apr 11;12(8):1614. doi: 10.3390/plants12081614. Plants (Basel). 2023. PMID: 37111838 Free PMC article.
-
Getting the most out of natural variation in C4 photosynthesis.Photosynth Res. 2014 Feb;119(1-2):157-67. doi: 10.1007/s11120-013-9872-8. Epub 2013 Jun 21. Photosynth Res. 2014. PMID: 23794170 Review.
-
A Brassica napus Reductase Gene Dissected by Associative Transcriptomics Enhances Plant Adaption to Freezing Stress.Front Plant Sci. 2020 Jun 26;11:971. doi: 10.3389/fpls.2020.00971. eCollection 2020. Front Plant Sci. 2020. PMID: 32676095 Free PMC article.
-
The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance.Plant Physiol. 2007 Nov;145(3):1006-17. doi: 10.1104/pp.107.103390. Epub 2007 Sep 7. Plant Physiol. 2007. PMID: 17827274 Free PMC article.
References
-
- Andrews TJ, Lorimer GH (1987) Rubisco: Structure, mechanisms, and prospects for improvement. In MD Hatch, NK Boardman, eds, The Biochemistry of Plants: A Comprehensive Treatise, Vol 10, Photosynthesis. Academic Press, New York, pp 131–218
-
- Badger MR, Andrews TJ (1987) Co-evolution of Rubisco and CO2 concentrating mechanisms. In J Biggins, ed, Progress in Photosynthesis Research, Vol 3. Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp 601–609
-
- Badger MR, Lorimer GH (1981) Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry 20: 2219–2225 - PubMed
-
- Berry JA, Farquhar GD (1978) The CO2 concentrating function of C4 photosynthesis: a biochemical model. In D Hall, J Coombs, T Goodwin, eds, The Proceedings of the Fourth International Congress on Photosynthesis. Biochemical Society of London, London, pp 119–131
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

