Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress
- PMID: 15665245
- PMCID: PMC1065371
- DOI: 10.1104/pp.104.054908
Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress
Abstract
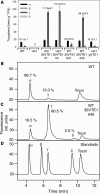
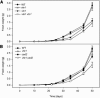
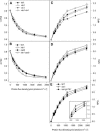
Tocopherol belongs to the Vitamin E class of lipid soluble antioxidants that are essential for human nutrition. In plants, tocopherol is synthesized in plastids where it protects membranes from oxidative degradation by reactive oxygen species. Tocopherol cyclase (VTE1) catalyzes the penultimate step of tocopherol synthesis, and an Arabidopsis (Arabidopsis thaliana) mutant deficient in VTE1 (vte1) is totally devoid of tocopherol. Overexpression of VTE1 resulted in an increase in total tocopherol of at least 7-fold in leaves, and a dramatic shift from alpha-tocopherol to gamma-tocopherol. Expression studies demonstrated that indeed VTE1 is a major limiting factor of tocopherol synthesis in leaves. Tocopherol deficiency in vte1 resulted in the increase in ascorbate and glutathione, whereas accumulation of tocopherol in VTE1 overexpressing plants led to a decrease in ascorbate and glutathione. Deficiency in one antioxidant in vte1, vtc1 (ascorbate deficient), or cad2 (glutathione deficient) led to increased oxidative stress and to the concomitant increase in alternative antioxidants. Double mutants of vte1 were generated with vtc1 and cad2. Whereas growth, chlorophyll content, and photosynthetic quantum yield were very similar to wild type in vte1, vtc1, cad2, or vte1vtc1, they were reduced in vte1cad2, indicating that the simultaneous loss of tocopherol and glutathione results in moderate oxidative stress that affects the stability and the efficiency of the photosynthetic apparatus.
Figures





Similar articles
-
Functional diversity of tocochromanols in plants.Planta. 2007 Jan;225(2):269-76. doi: 10.1007/s00425-006-0438-2. Epub 2006 Nov 18. Planta. 2007. PMID: 17115182 Review.
-
Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis.Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12495-500. doi: 10.1073/pnas.182330899. Epub 2002 Sep 4. Proc Natl Acad Sci U S A. 2002. PMID: 12213958 Free PMC article.
-
Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana.Biotechnol Lett. 2008 Jul;30(7):1275-80. doi: 10.1007/s10529-008-9672-y. Epub 2008 Mar 4. Biotechnol Lett. 2008. PMID: 18317702
-
Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana.Plant Physiol Biochem. 2009 May;47(5):384-90. doi: 10.1016/j.plaphy.2009.01.009. Epub 2009 Feb 12. Plant Physiol Biochem. 2009. PMID: 19264498
-
Plastochromanol-8: fifty years of research.Phytochemistry. 2014 Dec;108:9-16. doi: 10.1016/j.phytochem.2014.09.011. Epub 2014 Oct 9. Phytochemistry. 2014. PMID: 25308762 Review.
Cited by
-
Wide-genome QTL mapping of fruit quality traits in a tomato RIL population derived from the wild-relative species Solanum pimpinellifolium L.Theor Appl Genet. 2015 Oct;128(10):2019-35. doi: 10.1007/s00122-015-2563-4. Epub 2015 Jul 12. Theor Appl Genet. 2015. PMID: 26163766
-
Genetic basis for natural variation in seed vitamin E levels in Arabidopsis thaliana.Proc Natl Acad Sci U S A. 2006 Dec 5;103(49):18834-41. doi: 10.1073/pnas.0606221103. Epub 2006 Oct 31. Proc Natl Acad Sci U S A. 2006. PMID: 17077148 Free PMC article.
-
Functional diversity of tocochromanols in plants.Planta. 2007 Jan;225(2):269-76. doi: 10.1007/s00425-006-0438-2. Epub 2006 Nov 18. Planta. 2007. PMID: 17115182 Review.
-
Jasmonic Acid Seed Treatment Stimulates Insecticide Detoxification in Brassica juncea L.Front Plant Sci. 2018 Nov 2;9:1609. doi: 10.3389/fpls.2018.01609. eCollection 2018. Front Plant Sci. 2018. PMID: 30450109 Free PMC article.
-
ROS-Scavengers, Osmoprotectants and Violaxanthin De-Epoxidation in Salt-Stressed Arabidopsis thaliana with Different Tocopherol Composition.Int J Mol Sci. 2021 Oct 21;22(21):11370. doi: 10.3390/ijms222111370. Int J Mol Sci. 2021. PMID: 34768798 Free PMC article.
References
-
- Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ (1994) RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance gene. Science 265: 1856–1860 - PubMed
-
- Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis mutant deficient in γ-tocopherol methyltransferase. Plant Mol Biol 52: 1181–1190 - PubMed
-
- Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ (2003) Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol 21: 1082–1087 - PubMed
-
- Chan AC, Khai T, Raynor T, Ganz PR, Chow CK (1991) Regeneration of vitamin E in human platelets. J Biol Chem 266: 17290–17295 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

