Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization
- PMID: 15665832
- PMCID: PMC7095966
- DOI: 10.1038/nm1179
Angiotensin-converting enzyme is a GPI-anchored protein releasing factor crucial for fertilization
Abstract
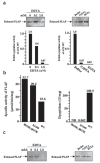
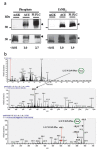
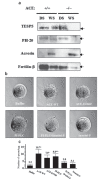
The angiotensin-converting enzyme (ACE) is a key regulator of blood pressure. It is known to cleave small peptides, such as angiotensin I and bradykinin and changes their biological activities, leading to upregulation of blood pressure. Here we describe a new activity for ACE: a glycosylphosphatidylinositol (GPI)-anchored protein releasing activity (GPIase activity). Unlike its peptidase activity, GPIase activity is weakly inhibited by the tightly binding ACE inhibitor and not inactivated by substitutions of core amino acid residues for the peptidase activity, suggesting that the active site elements for GPIase differ from those for peptidase activity. ACE shed various GPI-anchored proteins from the cell surface, and the process was accelerated by the lipid raft disruptor filipin. The released products carried portions of the GPI anchor, indicating cleavage within the GPI moiety. Further analysis by high-performance liquid chromatography-mass spectrometry predicted the cleavage site at the mannose-mannose linkage. GPI-anchored proteins such as TESP5 and PH-20 were released from the sperm membrane of wild-type mice but not in Ace knockout sperm in vivo. Moreover, peptidase-inactivated E414D mutant ACE and also PI-PLC rescued the egg-binding deficiency of Ace knockout sperms, implying that ACE plays a crucial role in fertilization through this activity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
ACE sets up fertilization.Nat Med. 2005 Feb;11(2):118-9. doi: 10.1038/nm0205-118. Nat Med. 2005. PMID: 15692590 No abstract available.
-
ACEing GPI release.Nat Struct Mol Biol. 2005 Feb;12(2):107-8. doi: 10.1038/nsmb0205-107. Nat Struct Mol Biol. 2005. PMID: 15702069 No abstract available.
-
Angiotensin-converting enzyme as a GPIase: a critical reevaluation.Nat Med. 2005 Nov;11(11):1139-40. doi: 10.1038/nm1105-1139. Nat Med. 2005. PMID: 16270062 No abstract available.
-
Male fertility is dependent on dipeptidase activity of testis ACE.Nat Med. 2005 Nov;11(11):1140-2; author reply 1142-3. doi: 10.1038/nm1105-1140. Nat Med. 2005. PMID: 16270063 No abstract available.
References
-
- Nozaki M, et al. Developmental abnormalities of glycosylphosphatidylinositol-anchor-deficient embryos revealed by Cre/loxP system. Lab. Invest. 1999;79:293–299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

