Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine
- PMID: 15665863
- PMCID: PMC1575998
- DOI: 10.1038/sj.bjp.0706074
Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine
Abstract
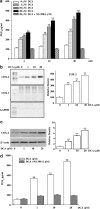
1. We investigated the role of deoxycholic acid in pacemaker currents using whole-cell patch-clamp techniques at 30 degrees C in cultured interstitial cells of Cajal (ICC) from murine small intestine. 2. The treatment of ICC with deoxycholic acid resulted in a decrease in the frequency and amplitude of pacemaker currents and increases in resting outward currents. Also, under current clamping, deoxycholic acid produced the hyperpolarization of membrane potential and decreased the amplitude of the pacemaker potentials. 3. These observed effects of deoxycholic acid on pacemaker currents and pacemaker potentials were completely suppressed by glibenclamide, an ATP-sensitive K(+) channel blocker. 4. NS-398, a specific cyclooxygenase-2 (COX-2) inhibitor, significantly inhibited the deoxycholic acid-induced effects. The treatment with prostaglandin E(2) (PGE(2)) led to a decrease in the amplitude and frequency of pacemaker currents and to an increase in resting outward currents, and these observed effects of PGE(2) were blocked by glibenclamide. 5. We next examined the role of deoxycholic acid in the production of PGE(2) in ICC, and found that deoxycholic acid increased PGE(2) production through the induction of COX-2 enzyme activity and its gene expression. 6. The results suggest that deoxycholic acid inhibits the pacemaker currents of ICC by activating ATP-sensitive K(+) channels through the production of PGE(2).
Figures






Similar articles
-
Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from the mouse small intestine.Naunyn Schmiedebergs Arch Pharmacol. 2007 Nov;376(3):175-84. doi: 10.1007/s00210-007-0187-1. Epub 2007 Oct 12. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17932655
-
Phentolamine inhibits the pacemaker activity of mouse interstitial cells of Cajal by activating ATP-sensitive K+ channels.Arch Pharm Res. 2010 Mar;33(3):479-89. doi: 10.1007/s12272-010-0319-x. Epub 2010 Mar 30. Arch Pharm Res. 2010. PMID: 20361315
-
Effects of pine needle extract on pacemaker currents in interstitial cells of Cajal from the murine small intestine.Mol Cells. 2005 Oct 31;20(2):235-40. Mol Cells. 2005. PMID: 16267398
-
Noradrenaline inhibits pacemaker currents through stimulation of beta 1-adrenoceptors in cultured interstitial cells of Cajal from murine small intestine.Br J Pharmacol. 2004 Feb;141(4):670-7. doi: 10.1038/sj.bjp.0705665. Epub 2004 Jan 26. Br J Pharmacol. 2004. PMID: 14744802 Free PMC article.
-
Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine.Cell Physiol Biochem. 2006;18(4-5):187-98. doi: 10.1159/000097516. Cell Physiol Biochem. 2006. PMID: 17167224
Cited by
-
Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from the mouse small intestine.Naunyn Schmiedebergs Arch Pharmacol. 2007 Nov;376(3):175-84. doi: 10.1007/s00210-007-0187-1. Epub 2007 Oct 12. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17932655
-
Ursodeoxycholic acid improves gastrointestinal motility defects in gallstone patients.World J Gastroenterol. 2006 Sep 7;12(33):5336-43. doi: 10.3748/wjg.v12.i33.5336. World J Gastroenterol. 2006. PMID: 16981264 Free PMC article. Clinical Trial.
-
Calcium-associated mechanisms in gut pacemaker activity.J Cell Mol Med. 2007 Sep-Oct;11(5):958-68. doi: 10.1111/j.1582-4934.2007.00107.x. J Cell Mol Med. 2007. PMID: 17979877 Free PMC article. Review.
-
Pituitary Adenylate Cyclase-activating Polypeptide Inhibits Pacemaker Activity of Colonic Interstitial Cells of Cajal.Korean J Physiol Pharmacol. 2015 Sep;19(5):435-40. doi: 10.4196/kjpp.2015.19.5.435. Epub 2015 Aug 20. Korean J Physiol Pharmacol. 2015. PMID: 26330756 Free PMC article.
-
Bile Acid Inhibition of N-type Calcium Channel Currents from Sympathetic Ganglion Neurons.Korean J Physiol Pharmacol. 2012 Feb;16(1):25-30. doi: 10.4196/kjpp.2012.16.1.25. Epub 2012 Feb 28. Korean J Physiol Pharmacol. 2012. PMID: 22416216 Free PMC article.
References
-
- AZZAROLI F., MAZZELLA G.F., MAZZEO C., SIMONI P., FESTI D., COLECCHIA A., MONTAGNANI M., MARTINO C., VILLANOVA N., RODA A., RODA E. Sluggish small bowel motility is involved in determining increased biliary deoxycholic acid in cholesterol gallstone patients. Am. J. gastroenterol. 1999;94:2453–2459. - PubMed
-
- BALISTRERI W.F., HEUBI J.E., SUCHY F.J. Bile acid metabolism: relationship of bile acid malabsorption and diarrhea. J. Pediatr. Gastroenterol. Nutr. 1983;2:105–121. - PubMed
-
- BOMZON A., LJUBUNCIC P. Bile acids as endogenous vasodilators. Bioche. Pharmacol. 1995;49:581–589. - PubMed
-
- EDWARDS G., WESTON A.H. The pharmacology of ATP-sensitive potassium channels. Annu. Rev. Pharmacol. Toxicol. 1993;33:597–637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

