Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury
- PMID: 15668136
- PMCID: PMC2581717
- DOI: 10.1016/j.ymthe.2004.09.013
Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury
Abstract
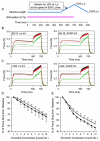
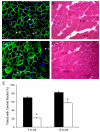
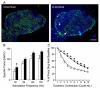
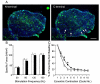
Duchenne muscular dystrophy (DMD) is the most common inherited lethal muscle degenerative disease. Currently there is no cure. Highly abbreviated microdystrophin cDNAs were developed recently for adeno-associated virus (AAV)-mediated DMD gene therapy. Among these, a C-terminal-truncated DeltaR4-R23/DeltaC microgene (DeltaR4/DeltaC) has been considered as a very promising therapeutic candidate gene. In this study, we packaged a CMV.DeltaR4/DeltaC cassette in AAV-5 and evaluated the transduction and muscle contractile profiles in the extensor digitorum longus muscles of young (7-week-old) and adult (9-month-old) mdx mice. At approximately 3 months post-gene transfer, 50-60% of the total myofibers were transduced in young mdx muscle and the percentage of centrally nucleated myofibers was reduced from approximately 70% in untreated mdx muscle to approximately 22% in microdystrophin-treated muscle. Importantly, this level of transduction protected mdx muscle from eccentric contraction-induced damage. In contrast, adult mdx muscle was more resistant to AAV-5 transduction, as only approximately 30% of the myofibers were transduced at 3 months postinfection. This transduction yielded marginal protection against eccentric contraction-induced injury. The extent of central nucleation was also more difficult to reverse in adult mdx muscle (from approximately 83% in untreated to approximately 58% in treated). Finally, we determined that the DeltaR4/DeltaC microdystrophin did not significantly alter the expression pattern of the endogenous full-length dystrophin in normal muscle. Neither did it have any adverse effects on normal muscle morphology or contractility. Taken together, our results suggest that AAV-mediated DeltaR4/DeltaC microdystrophin expression represents a promising approach to rescue muscular dystrophy in young mdx skeletal muscle.
Figures



References
-
- Emery AEH, Muntoni F. Duchenne Muscular Dystrophy. Oxford Univ. Press; Oxford/New York: 2003.
-
- Bushby KM, Hill A, Steele JG. Failure of early diagnosis in symptomatic Duchenne muscular dystrophy. Lancet. 1999;353:557–558. - PubMed
-
- Eagle M, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul. Disord. 2002;12:926–929. - PubMed
-
- Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Koenig M, et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

