Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles
- PMID: 15668297
- PMCID: PMC2171740
- DOI: 10.1083/jcb.200407078
Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles
Abstract
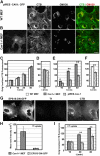
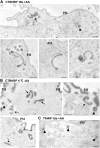
Using quantitative light microscopy and a modified immunoelectron microscopic technique, we have characterized the entry pathway of the cholera toxin binding subunit (CTB) in primary embryonic fibroblasts. CTB trafficking to the Golgi complex was identical in caveolin-1null (Cav1-/-) mouse embryonic fibroblasts (MEFs) and wild-type (WT) MEFs. CTB entry in the Cav1-/- MEFs was predominantly clathrin and dynamin independent but relatively cholesterol dependent. Immunoelectron microscopy was used to quantify budded and surface-connected caveolae and to identify noncaveolar endocytic vehicles. In WT MEFs, a small fraction of the total Cav1-positive structures were shown to bud from the plasma membrane (2% per minute), and budding increased upon okadaic acid or lactosyl ceramide treatment. However, the major carriers involved in initial entry of CTB were identified as uncoated tubular or ring-shaped structures. These carriers contained GPI-anchored proteins and fluid phase markers and represented the major vehicles mediating CTB uptake in both WT and caveolae-null cells.
Figures




References
-
- Anderson, R.G., B.A. Kamen, K.G. Rothberg, and S.W. Lacey. 1992. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 255:410–411. - PubMed
-
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303–1311. - PubMed
-
- Conner, S.D., and S.L. Schmid. 2003. Regulated portals of entry into the cell. Nature. 422:37–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

