Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae
- PMID: 15668298
- PMCID: PMC2171728
- DOI: 10.1083/jcb.200407113
Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae
Abstract
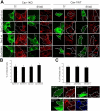
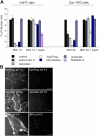
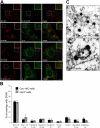
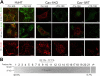
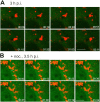
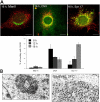
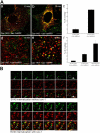
Simian Virus 40 (SV40) has been shown to enter host cells by caveolar endocytosis followed by transport via caveosomes to the endoplasmic reticulum (ER). Using a caveolin-1 (cav-1)-deficient cell line (human hepatoma 7) and embryonic fibroblasts from a cav-1 knockout mouse, we found that in the absence of caveolae, but also in wild-type embryonic fibroblasts, the virus exploits an alternative, cav-1-independent pathway. Internalization was rapid (t1/2 = 20 min) and cholesterol and tyrosine kinase dependent but independent of clathrin, dynamin II, and ARF6. The viruses were internalized in small, tight-fitting vesicles and transported to membrane-bounded, pH-neutral organelles similar to caveosomes but devoid of cav-1 and -2. The viruses were next transferred by microtubule-dependent vesicular transport to the ER, a step that was required for infectivity. Our results revealed the existence of a virus-activated endocytic pathway from the plasma membrane to the ER that involves neither clathrin nor caveolae and that can be activated also in the presence of cav-1.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

