Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation
- PMID: 15668399
- PMCID: PMC545492
- DOI: 10.1073/pnas.0406789102
Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation
Abstract
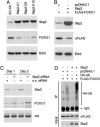
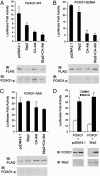
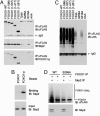
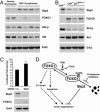
Forkhead transcription factors FOXO1 (FKHR), FOXO3a (FKHRL1), and FOXO4 (AFX) play a pivotal role in tumor suppression by inducing growth arrest and apoptosis. Loss of function of these factors due to phosphorylation and proteasomal degradation has been implicated in cell transformation and malignancy. However, the ubiquitin ligase necessary for the ubiquitination of the FOXO factors and the relevance of this regulation to tumorigenesis have not been characterized. Here we demonstrate that Skp2, an oncogenic subunit of the Skp1/Cul1/F-box protein ubiquitin complex, interacts with, ubiquitinates, and promotes the degradation of FOXO1. This effect of Skp2 requires Akt-specific phosphorylation of FOXO1 at Ser-256. Moreover, expression of Skp2 inhibits transactivation of FOXO1 and abolishes the inhibitory effect of FOXO1 on cell proliferation and survival. Furthermore, expression of the FOXO1 protein is lost in a mouse lymphoma model, where Skp2 is overexpressed. These data suggest that the Skp2-promoted proteolysis of FOXO1 plays a key role in tumorigenesis.
Figures

Similar articles
-
Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1-S transition.Oncogene. 2008 May 15;27(22):3176-85. doi: 10.1038/sj.onc.1210971. Epub 2007 Dec 10. Oncogene. 2008. PMID: 18071316
-
Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate.Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11515-20. doi: 10.1073/pnas.0603921103. Epub 2006 Jul 21. Proc Natl Acad Sci U S A. 2006. PMID: 16861300 Free PMC article.
-
PAX3-FKHR transformation increases 26 S proteasome-dependent degradation of p27Kip1, a potential role for elevated Skp2 expression.J Biol Chem. 2003 Jan 3;278(1):27-36. doi: 10.1074/jbc.M205424200. Epub 2002 Oct 24. J Biol Chem. 2003. PMID: 12401804
-
Regulation of FOXO protein stability via ubiquitination and proteasome degradation.Biochim Biophys Acta. 2011 Nov;1813(11):1961-4. doi: 10.1016/j.bbamcr.2011.01.007. Epub 2011 Jan 14. Biochim Biophys Acta. 2011. PMID: 21238503 Free PMC article. Review.
-
The role of Skp2 and its substrate CDKN1B (p27) in colorectal cancer.J Gastrointestin Liver Dis. 2015 Jun;24(2):225-34. doi: 10.15403/jgld.2014.1121.242.skp2. J Gastrointestin Liver Dis. 2015. PMID: 26114183 Review.
Cited by
-
Cellular and molecular biology of aging endothelial cells.J Mol Cell Cardiol. 2015 Dec;89(Pt B):122-35. doi: 10.1016/j.yjmcc.2015.01.021. Epub 2015 Feb 2. J Mol Cell Cardiol. 2015. PMID: 25655936 Free PMC article. Review.
-
Exploring the Genetic Conception of Obesity via the Dual Role of FoxO.Int J Mol Sci. 2021 Mar 20;22(6):3179. doi: 10.3390/ijms22063179. Int J Mol Sci. 2021. PMID: 33804729 Free PMC article. Review.
-
XBP-1u suppresses autophagy by promoting the degradation of FoxO1 in cancer cells.Cell Res. 2013 Apr;23(4):491-507. doi: 10.1038/cr.2013.2. Epub 2013 Jan 1. Cell Res. 2013. PMID: 23277279 Free PMC article.
-
Methylation by Set9 modulates FoxO3 stability and transcriptional activity.Aging (Albany NY). 2012 Jul;4(7):462-79. doi: 10.18632/aging.100471. Aging (Albany NY). 2012. PMID: 22820736 Free PMC article.
-
FOXO1 mediates the autocrine effect of endothelin-1 on endothelial cell survival.Mol Endocrinol. 2012 Jul;26(7):1213-24. doi: 10.1210/me.2011-1276. Epub 2012 May 8. Mol Endocrinol. 2012. PMID: 22570335 Free PMC article.
References
-
- Medema, R. H., Kops, G. J., Bos, J. L. & Burgering, B. M. (2000) Nature 404, 782–787. - PubMed
-
- Ramaswamy, S., Nakamura, N., Sansal, I., Bergeron, L. & Sellers, W. R. (2002) Cancer Cell 2, 81–91. - PubMed
-
- Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. C., Blenis, J. & Greenberg, M. E. (1999) Cell 96, 857–868. - PubMed
-
- Modur, V., Nagarajan, R., Evers, B. M. & Milbrandt, J. (2002) J. Biol. Chem. 277, 47928–47937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

