A randomised factorial trial of sequential doxorubicin and CMF vs CMF and chemotherapy alone vs chemotherapy followed by goserelin plus tamoxifen as adjuvant treatment of node-positive breast cancer
- PMID: 15668708
- PMCID: PMC2362097
- DOI: 10.1038/sj.bjc.6602355
A randomised factorial trial of sequential doxorubicin and CMF vs CMF and chemotherapy alone vs chemotherapy followed by goserelin plus tamoxifen as adjuvant treatment of node-positive breast cancer
Abstract
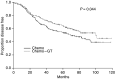
The sequential doxorubicin --> CMF (CMF=cyclophosphamide, methotrexate, fluorouracil) regimen has never been compared to CMF in a randomised trial. The role of adding goserelin and tamoxifen after chemotherapy is unclear. In all, 466 premenopausal node-positive patients were randomised to: (a) CMF x 6 cycles (CMF); (b) doxorubicin x 4 cycles followed by CMF x 6 cycles (A --> CMF); (c) CMF x 6 cycles followed by goserelin plus tamoxifen x 2 years (CMF --> GT); and (d) doxorubicin x 4 cycles followed by CMF x 6 cycles followed by goserelin plus tamoxifen x 2 years (A --> CMF --> GT). The study used a 2 x 2 factorial experimental design to assess: (1) the effect of the chemotherapy regimens (CMF vs A --> CMF or arms a+c vs b+d) and (2) the effect of adding GT after chemotherapy (arms a+b vs c+d). At a median follow-up of 72 months, A --> CMF as compared to CMF significantly improved disease-free survival (DFS) with a multivariate hazard ratio (HR)=0.740 (95% confidence interval (CI): 0.556-0.986; P=0.040) and produced a nonsignificant improvement of overall survival (OS) (HR=0.764; 95% CI: 0.489-1.193). The addition of GT after chemotherapy significantly improved DFS (HR=0.74; 95% CI: 0.555-0.987; P=0.040), with a nonsignificant improvement of OS (HR=0.84; 95% CI: 0.54-1.32). A --> CMF is superior to CMF. Adding GT after chemotherapy is beneficial for premenopausal node-positive patients.
Figures
References
-
- Baum M, Houghton J, Olding-Smee W et al (2001) On behalf of the ZIPP Group. Adjuvant Zoladex in premenopausal patients with early breast cancer: results from ZIPP trial (abs P64). Breast 10(Suppl 1): S32–S33
-
- Boccardo F, Rubagotti A, Amoroso D, Mesiti M, Romeo D, Sismondi P, Giai M, Genta F, Pacini P, Distante V, Bolognesi A, Aldrighetti D, Farris A (2000) Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-/perimenopausal breast cancer patients: results of the Italian Breast Cancer Adjuvant Study Group 02 randomized trial. J Clin Oncol 18: 2718–2727 - PubMed
-
- Bonadonna G, Zambetti M, Valagussa P (1995) Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results. JAMA 273: 542–547 - PubMed
-
- Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, Ferree CR, Muss HB, Green MR, Norton L, Frei III E (1998) Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 90: 1205–1211 - PubMed
-
- Buzzoni R, Bonadonna G, Valagussa P, Zambetti M (1991) Adjuvant chemotherapy with doxorubicin plus cyclophosphamide, methotrexate, and fluorouracil in the treatment of resectable breast cancer with more than three positive axillary nodes. J Clin Oncol 9: 2134–2140 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous