IL-1 mediates TNF-induced osteoclastogenesis
- PMID: 15668736
- PMCID: PMC544608
- DOI: 10.1172/JCI23394
IL-1 mediates TNF-induced osteoclastogenesis
Abstract
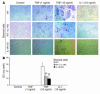
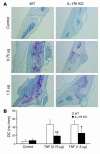
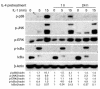
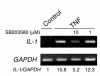
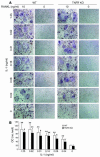
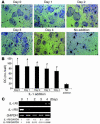
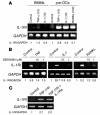
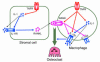
TNF-induced receptor activator NF-kappaB ligand (RANKL) synthesis by bone marrow stromal cells is a fundamental component of inflammatory osteolysis. We found that this process was abolished by IL-1 receptor antagonist (IL-1Ra) or in stromal cells derived from type I IL-1 receptor-deficient (IL-1RI-deficient) mice. Reflecting sequential signaling of the cytokines TNF and IL-1, TNF induces stromal cell expression of IL-1 and IL-1RI. These data suggest that TNF regulates RANKL expression via IL-1, and, therefore, IL-1 plays a role in TNF-induced periarticular osteolysis. Consistent with this posture, TNF-stimulated osteoclastogenesis in cultures consisting of WT marrow macrophages and stromal cells exposed to IL-1Ra or in cocultures established with IL-1RI-deficient stromal cells was reduced approximately 50%. The same magnitude of osteoclast inhibition occurred in IL-1RI-deficient mice following TNF administration in vivo. Like TNF, IL-1 directly targeted osteoclast precursors and promoted the osteoclast phenotype in a TNF-independent manner in the presence of permissive levels of RANKL. IL-1 is able to induce RANKL expression by stromal cells and directly stimulate osteoclast precursor differentiation under the aegis of p38 MAPK. Thus, IL-1 mediates the osteoclastogenic effect of TNF by enhancing stromal cell expression of RANKL and directly stimulating differentiation of osteoclast precursors.
Figures





Similar articles
-
MKK3/6-p38 MAPK signaling is required for IL-1beta and TNF-alpha-induced RANKL expression in bone marrow stromal cells.J Interferon Cytokine Res. 2006 Oct;26(10):719-29. doi: 10.1089/jir.2006.26.719. J Interferon Cytokine Res. 2006. PMID: 17032166
-
NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis.J Bone Miner Res. 2002 Jul;17(7):1200-10. doi: 10.1359/jbmr.2002.17.7.1200. J Bone Miner Res. 2002. PMID: 12096833
-
Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo.J Immunol. 2004 Oct 15;173(8):4838-46. doi: 10.4049/jimmunol.173.8.4838. J Immunol. 2004. PMID: 15470024
-
A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function.Biochem Biophys Res Commun. 1999 Mar 24;256(3):449-55. doi: 10.1006/bbrc.1999.0252. Biochem Biophys Res Commun. 1999. PMID: 10080918 Review.
-
The molecular basis of osteoclast differentiation and activation.Novartis Found Symp. 2001;232:235-47; discussion 247-50. doi: 10.1002/0470846658.ch16. Novartis Found Symp. 2001. PMID: 11277084 Review.
Cited by
-
A Review of Signaling Transduction Mechanisms in Osteoclastogenesis Regulation by Autophagy, Inflammation, and Immunity.Int J Mol Sci. 2022 Aug 30;23(17):9846. doi: 10.3390/ijms23179846. Int J Mol Sci. 2022. PMID: 36077242 Free PMC article. Review.
-
Osteoblast role in the pathogenesis of rheumatoid arthritis.Mol Biol Rep. 2021 Mar;48(3):2843-2852. doi: 10.1007/s11033-021-06288-y. Epub 2021 Mar 27. Mol Biol Rep. 2021. PMID: 33774802 Free PMC article. Review.
-
The IVVY Motif and Tumor Necrosis Factor Receptor-associated Factor (TRAF) Sites in the Cytoplasmic Domain of the Receptor Activator of Nuclear Factor κB (RANK) Cooperate to Induce Osteoclastogenesis.J Biol Chem. 2015 Sep 25;290(39):23738-50. doi: 10.1074/jbc.M115.667535. Epub 2015 Aug 14. J Biol Chem. 2015. PMID: 26276390 Free PMC article.
-
Role of the immune system in postmenopausal bone loss.Curr Osteoporos Rep. 2005 Sep;3(3):92-7. doi: 10.1007/s11914-005-0016-8. Curr Osteoporos Rep. 2005. PMID: 16131428 Review.
-
The role played by ailanthone in inhibiting bone metastasis of breast cancer by regulating tumor-bone microenvironment through the RANKL-dependent pathway.Front Pharmacol. 2023 Jan 5;13:1081978. doi: 10.3389/fphar.2022.1081978. eCollection 2022. Front Pharmacol. 2023. PMID: 36686653 Free PMC article.
References
-
- Teitelbaum SL. Bone resorption by osteo-clasts. Science. 2000;289:1504–1508. - PubMed
-
- Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J. Cell. Biochem. 2003;89:180–190. - PubMed
-
- Feldmann M, Maini RN. Anti-TNF therapy of rheumatoid arthritis: what have we learned? Ann. Rev. Immunol. 2001;19:163–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR32788/AR/NIAMS NIH HHS/United States
- AR46852/AR/NIAMS NIH HHS/United States
- R01 AR046523/AR/NIAMS NIH HHS/United States
- R37 AR046523/AR/NIAMS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 AR046852/AR/NIAMS NIH HHS/United States
- AR48812/AR/NIAMS NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- R01 AR048812/AR/NIAMS NIH HHS/United States
- R01 AR048853/AR/NIAMS NIH HHS/United States
- AR46523/AR/NIAMS NIH HHS/United States
- AR48853/AR/NIAMS NIH HHS/United States
- DK56341/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

