Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9
- PMID: 15668740
- PMCID: PMC544604
- DOI: 10.1172/JCI23025
Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9
Abstract
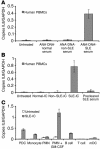
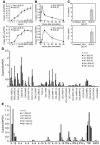
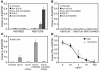
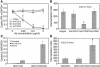
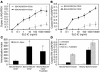
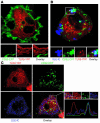
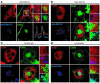
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by pathogenic autoantibodies against nucleoproteins and DNA. Here we show that DNA-containing immune complexes (ICs) within lupus serum (SLE-ICs), but not protein-containing ICs from other autoimmune rheumatic diseases, stimulates plasmacytoid DCs (PDCs) to produce cytokines and chemokines via a cooperative interaction between Toll-like receptor 9 (TLR9) and FcgammaRIIa (CD32). SLE-ICs transiently colocalized to a subcellular compartment containing CD32 and TLR9, and CD32+, but not CD32-, PDCs internalized and responded to SLE-ICs. Our findings demonstrate a novel functional interaction between Fc receptors and TLRs, defining a pathway in which CD32 delivers SLE-ICs to intracellular lysosomes containing TLR9, inducing a signaling cascade leading to PDC activation. These data demonstrate that endogenous DNA-containing autoantibody complexes found in the serum of patients with SLE activate the innate immune system and suggest a novel mechanism whereby these ICs contribute to the pathogenesis of this autoimmune disease.
Figures

References
-
- Lawrence RC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. - PubMed
-
- Bootsma H, et al. Anti-double stranded DNA antibodies in systemic lupus erythematosus: detection and clinical relevance of IgM-class antibodies. Scand. J. Rheumatol. 1996;25:352–359. - PubMed
-
- Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. - PubMed
-
- ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33:634–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

