Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells
- PMID: 15669919
- PMCID: PMC1138953
- DOI: 10.1042/BJ20041682
Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells
Abstract
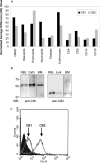
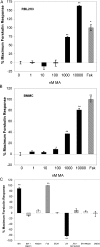
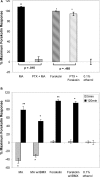
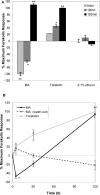
Cannabinoids are broadly immunosuppressive, and anti-inflammatory properties have been reported for certain marijuana constituents and endogenously produced cannabinoids. The CB2 cannabinoid receptor is an established constituent of immune system cells, and we have recently established that the CB1 cannabinoid receptor is expressed in mast cells. In the present study, we sought to define a role for CB1 in mast cells and to identify the signalling pathways that may mediate the suppressive effects of CB1 ligation on mast cell activation. Our results show that CB1 and CB2 mediate diametrically opposed effects on cAMP levels in mast cells. The observed long-term stimulation of cAMP levels by the Galpha(i/o)-coupled CB1 is paradoxical, and our results indicate that it may be attributed to CB1-mediated transcriptional regulation of specific adenylate cyclase isoenzymes that exhibit superactivatable kinetics. Taken together, these results reveal the complexity in signalling of natively co-expressed cannabinoid receptors and suggest that some anti-inflammatory effects of CB1 ligands may be attributable to sustained cAMP elevation that, in turn, causes suppression of mast cell degranulation.
Figures

Similar articles
-
Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: endogenous signal transduction pathways.Biochem Pharmacol. 2005 Jun 15;69(12):1705-13. doi: 10.1016/j.bcp.2005.03.027. Biochem Pharmacol. 2005. PMID: 15885656
-
Virodhamine and CP55,940 modulate cAMP production and IL-8 release in human bronchial epithelial cells.Br J Pharmacol. 2007 Aug;151(7):1041-8. doi: 10.1038/sj.bjp.0707320. Epub 2007 Jun 11. Br J Pharmacol. 2007. PMID: 17558435 Free PMC article.
-
Evaluation of selective cannabinoid CB(1) and CB(2) receptor agonists in a mouse model of lipopolysaccharide-induced interstitial cystitis.Eur J Pharmacol. 2014 Apr 15;729:67-74. doi: 10.1016/j.ejphar.2014.02.013. Epub 2014 Feb 20. Eur J Pharmacol. 2014. PMID: 24561047
-
Cannabinoid signalling.Life Sci. 2006 Jan 2;78(6):549-63. doi: 10.1016/j.lfs.2005.05.055. Epub 2005 Aug 18. Life Sci. 2006. PMID: 16109430 Review.
-
Cannabinoid receptor signaling.Handb Exp Pharmacol. 2005;(168):53-79. doi: 10.1007/3-540-26573-2_2. Handb Exp Pharmacol. 2005. PMID: 16596771 Review.
Cited by
-
Multi-omics analysis reveals the metabolic regulators of duodenal low-grade inflammation in a functional dyspepsia model.Front Immunol. 2022 Aug 24;13:944591. doi: 10.3389/fimmu.2022.944591. eCollection 2022. Front Immunol. 2022. PMID: 36091013 Free PMC article.
-
Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor.Nutrients. 2022 Dec 30;15(1):178. doi: 10.3390/nu15010178. Nutrients. 2022. PMID: 36615835 Free PMC article.
-
Neurogenic Inflammation in the Context of Endometriosis-What Do We Know?Int J Mol Sci. 2021 Dec 3;22(23):13102. doi: 10.3390/ijms222313102. Int J Mol Sci. 2021. PMID: 34884907 Free PMC article. Review.
-
Endo-cannabinoids system and the toxicity of cannabinoids with a biotechnological approach.EXCLI J. 2017 May 15;16:688-711. doi: 10.17179/excli2017-257. eCollection 2017. EXCLI J. 2017. PMID: 28827985 Free PMC article. Review.
-
The CB2 receptor and its role as a regulator of inflammation.Cell Mol Life Sci. 2016 Dec;73(23):4449-4470. doi: 10.1007/s00018-016-2300-4. Epub 2016 Jul 11. Cell Mol Life Sci. 2016. PMID: 27402121 Free PMC article. Review.
References
-
- Galli S. J. Mast cells and basophils. Curr. Opin. Hematol. 2000;7:32–39. - PubMed
-
- Galli S. J., Wedemeyer J., Tsai M. Analyzing the roles of mast cells and basophils in host defense and other biological responses. Int. J. Hematol. 2002;75:363–369. - PubMed
-
- Roth M. D., Arora A., Barsky S. H., Kleerup E. C., Simmons M., Tashkin D. P. Airway inflammation in young marijuana and tobacco smokers. Am. J. Respir. Crit. Care Med. 1998;157:928–937. - PubMed
-
- Sharma B. B., Apgar J. R., Liu F. T. Mast cells. Receptors, secretagogues, and signaling. Clin. Rev. Allergy Immunol. 2002;22:119–148. - PubMed
-
- Wedemeyer J., Tsai M., Galli S. J. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 2000;12:624–631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

