Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures
- PMID: 15670336
- PMCID: PMC548670
- DOI: 10.1186/1742-2094-2-4
Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures
Abstract
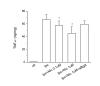
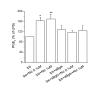
BACKGROUND: Nicotinic acetylcholine (Ach) receptors are ligand-gated pentameric ion channels whose main function is to transmit signals for the neurotransmitter Ach in peripheral and central nervous system. However, the alpha7 nicotinic receptor has been recently found in several non-neuronal cells and described as an important regulator of cellular function. Nicotine and ACh have been recently reported to inhibit tumor necrosis factor-alpha (TNF-alpha) production in human macrophages as well as in mouse microglial cultures. In the present study, we investigated whether the stimulation of alpha7 nicotinic receptor by the specific agonist nicotine could affect the functional state of activated microglia by promoting and/or inhibiting the release of other important pro-inflammatory and lipid mediator such as prostaglandin E2. METHODS: Expression of alpha7 nicotinic receptor in rat microglial cell was examined by RT-PCR, immunofluorescence staining and Western blot. The functional effects of alpha7 receptor activation were analyzed in resting or lipopolysaccharide (LPS) stimulated microglial cells pre-treated with nicotine. Culture media were assayed for the levels of tumor necrosis factor, interleukin-1beta, nitric oxide, interleukin-10 and prostaglandin E2. Total RNA was assayed by RT-PCR for the expression of COX-2 mRNA. RESULTS: Rat microglial cells express alpha7 nicotinic receptor, and its activation by nicotine dose-dependently reduces the LPS-induced release of TNF-alpha, but has little or no effect on nitric oxide, interleukin-10 and interleukin-1beta. By contrast, nicotine enhances the expression of cyclooxygenase-2 and the synthesis of one of its major products, prostaglandin E2. CONCLUSIONS: Since prostaglandin E2 modulates several macrophage and lymphocyte functions, which are instrumental for inflammatory resolution, our study further supports the existence of a brain cholinergic anti-inflammatory pathway mediated by alpha7 nicotinic receptor that could be potentially exploited for novel treatments of several neuropathologies in which local inflammation, sustained by activated microglia, plays a crucial role.
Figures
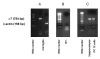


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

