Enthalpy of helix-coil transition: missing link in rationalizing the thermodynamics of helix-forming propensities of the amino acid residues
- PMID: 15671166
- PMCID: PMC547846
- DOI: 10.1073/pnas.0408004102
Enthalpy of helix-coil transition: missing link in rationalizing the thermodynamics of helix-forming propensities of the amino acid residues
Abstract
It is known that different amino acid residues have effects on the thermodynamic stability of an alpha-helix. The underlying mechanism for the thermodynamic helical propensity is not well understood. The major accepted hypothesis is the difference in the side-chain configurational entropy loss upon helix formation. However, the changes in the side-chain configurational entropy explain only part of the thermodynamic helical propensity, thus implying that there must be a difference in the enthalpy of helix-coil transition for different residues. This work provides an experimental test to this hypothesis. Direct calorimetric measurements of folding of a model host peptide in which the helix formation is induced by metal binding is applied to a wide range of residue types, both naturally occurring and nonnatural, at the guest site. Based on the calorimetric results for 12 peptides, it was found that indeed there is a difference in the enthalpy of helix-coil transition for different amino acid residues, and simple empirical rules that define these differences are presented. The obtained difference in the enthalpies of helix-coil transition complement the differences in configurational entropies and provide the complete thermodynamic characterization of the helix-forming tendencies.
Figures

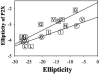
 , P2T. The lines through the points are shown only to guide the eye.
, P2T. The lines through the points are shown only to guide the eye.
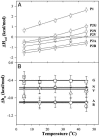
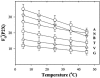
 , P1.
, P1.
Similar articles
-
A calorimetric study of the folding-unfolding of an alpha-helix with covalently closed N and C-terminal loops.J Mol Biol. 1999 Aug 27;291(4):965-76. doi: 10.1006/jmbi.1999.3025. J Mol Biol. 1999. PMID: 10452900
-
Helix propensities of the amino acids measured in alanine-based peptides without helix-stabilizing side-chain interactions.Protein Sci. 1994 May;3(5):843-52. doi: 10.1002/pro.5560030514. Protein Sci. 1994. PMID: 8061613 Free PMC article.
-
Entropy reduction in unfolded peptides (and proteins) due to conformational preferences of amino acid residues.Phys Chem Chem Phys. 2014 Nov 7;16(41):22527-36. doi: 10.1039/c4cp02108h. Phys Chem Chem Phys. 2014. PMID: 25227444
-
Helix design, prediction and stability.Curr Opin Biotechnol. 1995 Aug;6(4):382-6. doi: 10.1016/0958-1669(95)80066-2. Curr Opin Biotechnol. 1995. PMID: 7579647 Review.
-
Thermodynamics Of alpha-Helix Formation.Adv Protein Chem. 2005;72:199-226. doi: 10.1016/S0065-3233(05)72008-8. Adv Protein Chem. 2005. PMID: 16581378 Review.
Cited by
-
Uncovering the Contributions of Charge Regulation to the Stability of Single Alpha Helices.Chemphyschem. 2023 Apr 3;24(7):e202200746. doi: 10.1002/cphc.202200746. Epub 2023 Jan 4. Chemphyschem. 2023. PMID: 36599672 Free PMC article.
-
Capping parallel β-sheets of acetyl(Ala)6NH2 with an acetyl(Ala)5ProNH2 can arrest the growth of the sheet, suggesting a potential for curtailing amyloid growth. An ONIOM and density functional theory study.Biochemistry. 2014 Feb 4;53(4):617-23. doi: 10.1021/bi401366w. Epub 2014 Jan 23. Biochemistry. 2014. PMID: 24422496 Free PMC article.
-
Nucleation and stability of hydrogen-bond surrogate-based alpha-helices.Org Biomol Chem. 2006 Nov 21;4(22):4074-81. doi: 10.1039/b612891b. Org Biomol Chem. 2006. PMID: 17312961 Free PMC article.
-
Unraveling the Thermodynamics of Ultra-tight Binding of Intrinsically Disordered Proteins.Front Mol Biosci. 2021 Aug 31;8:726824. doi: 10.3389/fmolb.2021.726824. eCollection 2021. Front Mol Biosci. 2021. PMID: 34532345 Free PMC article.
-
A backbone-based theory of protein folding.Proc Natl Acad Sci U S A. 2006 Nov 7;103(45):16623-33. doi: 10.1073/pnas.0606843103. Epub 2006 Oct 30. Proc Natl Acad Sci U S A. 2006. PMID: 17075053 Free PMC article. Review.
References
-
- Scholtz, J. M. & Baldwin, R. L. (1992) Annu. Rev. Biophys. Biomol. Struct. 21, 95-118. - PubMed
-
- Chakrabartty, A. & Baldwin, R. L. (1995) Adv. Protein Chem. 46, 141-176. - PubMed
-
- Rohl, C. A. & Baldwin, R. L. (1998) Methods Enzymol. 295, 1-26. - PubMed
-
- Baldwin, R. L. & Rose, G. D. (1999) Trends Biochem. Sci. 24, 26-33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

