Enthalpy of helix-coil transition: missing link in rationalizing the thermodynamics of helix-forming propensities of the amino acid residues
- PMID: 15671166
- PMCID: PMC547846
- DOI: 10.1073/pnas.0408004102
Enthalpy of helix-coil transition: missing link in rationalizing the thermodynamics of helix-forming propensities of the amino acid residues
Abstract
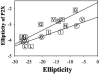
It is known that different amino acid residues have effects on the thermodynamic stability of an alpha-helix. The underlying mechanism for the thermodynamic helical propensity is not well understood. The major accepted hypothesis is the difference in the side-chain configurational entropy loss upon helix formation. However, the changes in the side-chain configurational entropy explain only part of the thermodynamic helical propensity, thus implying that there must be a difference in the enthalpy of helix-coil transition for different residues. This work provides an experimental test to this hypothesis. Direct calorimetric measurements of folding of a model host peptide in which the helix formation is induced by metal binding is applied to a wide range of residue types, both naturally occurring and nonnatural, at the guest site. Based on the calorimetric results for 12 peptides, it was found that indeed there is a difference in the enthalpy of helix-coil transition for different amino acid residues, and simple empirical rules that define these differences are presented. The obtained difference in the enthalpies of helix-coil transition complement the differences in configurational entropies and provide the complete thermodynamic characterization of the helix-forming tendencies.
Figures

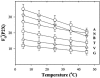
 , P2T. The lines through the points are shown only to guide the eye.
, P2T. The lines through the points are shown only to guide the eye.
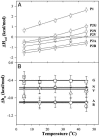
 , P1.
, P1.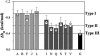
References
-
- Scholtz, J. M. & Baldwin, R. L. (1992) Annu. Rev. Biophys. Biomol. Struct. 21, 95-118. - PubMed
-
- Chakrabartty, A. & Baldwin, R. L. (1995) Adv. Protein Chem. 46, 141-176. - PubMed
-
- Rohl, C. A. & Baldwin, R. L. (1998) Methods Enzymol. 295, 1-26. - PubMed
-
- Baldwin, R. L. & Rose, G. D. (1999) Trends Biochem. Sci. 24, 26-33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

