Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells
- PMID: 15671173
- PMCID: PMC545583
- DOI: 10.1073/pnas.0401851102
Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells
Abstract
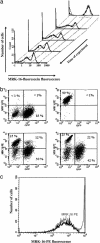
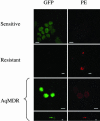
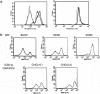
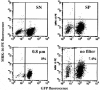
The overexpression of P-glycoprotein (P-gp) causes resistance to chemotherapy in many tumor types. Here, we report intercellular transfer of functional P-gp from P-gp-positive to P-gp-negative cells in vitro and in vivo. The expression of acquired P-gp is transient in isolated cells but persists in the presence of P-gp-positive cells or under the selective pressure of colchicine. The intercellular transfer of functional P-gp occurs between different tumor cell types and results in increased drug resistance both in vitro and in vivo. Most importantly, the acquired resistance permits tumor cells to survive potentially toxic drug concentrations long enough to develop intrinsic P-gp-mediated resistance. P-gp transfer also occurs to putative components of tumor stroma, such as fibroblasts, raising the possibility that multidrug resistance could be conferred by resistant tumor cells to critical stromal elements within the tumor mass. This is the first report, to our knowledge, that a protein transferred between cells retains its function and confers a complex biologic property upon the recipient cell. These findings have important implications for proteomic analyses in tumor samples and resistance to cancer therapy.
Figures


Similar articles
-
Actin disruption inhibits endosomal traffic of P-glycoprotein-EGFP and resistance to daunorubicin accumulation.Am J Physiol Cell Physiol. 2007 Apr;292(4):C1543-52. doi: 10.1152/ajpcell.00068.2006. Epub 2006 Nov 22. Am J Physiol Cell Physiol. 2007. PMID: 17122416
-
Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer.Cancer Res. 2006 May 1;66(9):4808-15. doi: 10.1158/0008-5472.CAN-05-3322. Cancer Res. 2006. PMID: 16651436 Free PMC article.
-
The anti-estrogen receptor drug, tamoxifen, is selectively Lethal to P-glycoprotein-expressing Multidrug resistant tumor cells.BMC Cancer. 2023 Jan 6;23(1):24. doi: 10.1186/s12885-022-10474-x. BMC Cancer. 2023. PMID: 36609245 Free PMC article.
-
Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer.Nanomedicine. 2022 Feb;40:102494. doi: 10.1016/j.nano.2021.102494. Epub 2021 Nov 12. Nanomedicine. 2022. PMID: 34775061 Review.
-
The role of P-glycoprotein in drug resistance in multiple myeloma.Leuk Lymphoma. 2015 Jan;56(1):26-33. doi: 10.3109/10428194.2014.907890. Epub 2014 Jun 5. Leuk Lymphoma. 2015. PMID: 24678978 Review.
Cited by
-
Breast cancer-derived microparticles display tissue selectivity in the transfer of resistance proteins to cells.PLoS One. 2013 Apr 12;8(4):e61515. doi: 10.1371/journal.pone.0061515. Print 2013. PLoS One. 2013. PMID: 23593486 Free PMC article.
-
Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities.Adv Drug Deliv Rev. 2013 Nov;65(13-14):1731-47. doi: 10.1016/j.addr.2013.09.001. Epub 2013 Sep 10. Adv Drug Deliv Rev. 2013. PMID: 24036273 Free PMC article. Review.
-
Different Ability of Multidrug-Resistant and -Sensitive Counterpart Cells to Release and Capture Extracellular Vesicles.Cells. 2021 Oct 26;10(11):2886. doi: 10.3390/cells10112886. Cells. 2021. PMID: 34831110 Free PMC article.
-
Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours.PLoS One. 2008;3(12):e3894. doi: 10.1371/journal.pone.0003894. Epub 2008 Dec 16. PLoS One. 2008. PMID: 19079610 Free PMC article.
-
Growth Hormone Upregulates Melanoma Drug Resistance and Migration via Melanoma-Derived Exosomes.Cancers (Basel). 2024 Jul 24;16(15):2636. doi: 10.3390/cancers16152636. Cancers (Basel). 2024. PMID: 39123364 Free PMC article.
References
-
- Darland, D. C. & D'Amore, P. A. (2001) Curr. Top. Dev. Biol. 52, 107–149. - PubMed
-
- Ambudkar, S. V., Dey, S., Hrycyna, C. A., Ramachandra, M., Pastan, I. & Gottesman, M. M. (1999) Annu. Rev. Pharmacol. Toxicol. 39, 361–398. - PubMed
-
- Bellamy, W. T. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 161–183. - PubMed
-
- Trock, B. J., Leonessa, F. & Clarke, R. (1997) J. Natl. Cancer Inst. 89, 917–931. - PubMed
-
- Dunne, B. M., Mc Namara, M., Clynes, M., Schering, S. G., Larkin, A. M., Moran, E., Barnes, C. & Kennedy, S. M. (1998) Hum. Pathol. 29, 594–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

