Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile, Thermus thermophilus
- PMID: 15673283
- PMCID: PMC1138949
- DOI: 10.1042/BJ20041778
Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile, Thermus thermophilus
Abstract
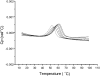
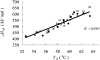
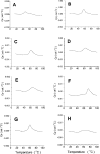
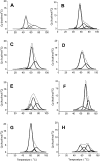
Extreme thermophiles produce two types of unusual polyamine: long linear polyamines such as caldopentamine and caldohexamine, and branched polyamines such as quaternary ammonium compounds [e.g. tetrakis(3-aminopropyl)ammonium]. To clarify the physiological roles of long linear and branched polyamines in thermophiles, we synthesized them chemically and tested their effects on the stability of ds (double-stranded) and ss (single-stranded) DNAs and tRNA in response to thermal denaturation, as measured by differential scanning calorimetry. Linear polyamines stabilized dsDNA in proportion to the number of amino nitrogen atoms within their molecular structure. We used the empirical results to derive formulae that estimate the melting temperature of dsDNA in the presence of polyamines of a particular molecular composition. ssDNA and tRNA were stabilized more effectively by tetrakis(3-aminopropyl)ammonium than any of the other polyamines tested. We propose that long linear polyamines are effective to stabilize DNA, and tetrakis(3-aminopropyl)ammonium plays important roles in stabilizing RNAs in thermophile cells.
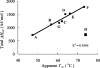
Figures



References
-
- Tabor C. W., Tabor H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. - PubMed
-
- Cohen S. S. New York: Oxford University Press; 1998. A Guide to the Polyamines.
-
- Yoshida M., Kashiwagi K., Kawai G., Ishihama A., Igarashi K. Polyamine enhancement of the synthesis of adenylate cyclase at the translational level and the consequential stimulation of the synthesis of the RNA polymerase σ28 subunit. J. Biol. Chem. 2001;276:16289–16295. - PubMed
-
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

