Inhibition of the central extended amygdala by loud noise and restraint stress
- PMID: 15673443
- PMCID: PMC2430886
- DOI: 10.1111/j.1460-9568.2005.03865.x
Inhibition of the central extended amygdala by loud noise and restraint stress
Abstract
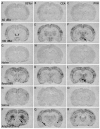
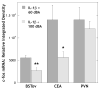
It is well established that the central nucleus of the amygdala (CEA) is involved in responses to stress, fear and anxiety. Many studies have used c-fos expression to map the brain's response to processive stress, but curiously the CEA generally is not highly activated. We have previously shown that exposure to a novel vs. home environment reduces amphetamine-induced activation of the lateral CEA (CEAl) and the oval nucleus of the bed nucleus of the stria terminalis (BSTov). This is consistent with the idea that processive stress inhibits neurons in these nuclei. We have tested this hypothesis by exposing rats to noise, at a range of intensities from non-stressful to stressful, or to restraint conditions, immediately after a remote injection of amphetamine, 2 mg/kg i.p., or interleukin-1beta (IL-1beta) 0.5 microg/kg i.p. (used to obtain a level of c-fos mRNA against which to measure inhibition). In keeping with our hypothesis, amphetamine- or IL-1beta-induced c-fos and zif-268 mRNA were significantly decreased in the CEAl and BSTov under conditions of loud noise or restraint stress compared with control conditions. This inhibition does not require a stress-induced rise in corticosterone because data were similar in animals that had been adrenalectomized with a low-dose corticosterone replacement. As both the CEAl and BSTov are highly gamma-aminobutyric acid (GABA) -ergic and project to the medial CEA (CEAm), their inhibition potentially causes an increased input to the CEAm. As the CEAm is a major output nucleus of the amygdala, this could have important consequences within the neural circuitry controlling responses to processive stress.
Figures
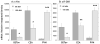
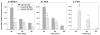
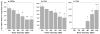



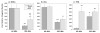
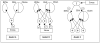
Similar articles
-
Conditioned fear inhibits c-fos mRNA expression in the central extended amygdala.Brain Res. 2008 Sep 10;1229:137-46. doi: 10.1016/j.brainres.2008.06.085. Epub 2008 Jul 2. Brain Res. 2008. PMID: 18634767 Free PMC article.
-
Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta.J Comp Neurol. 1999 Oct 11;413(1):113-28. J Comp Neurol. 1999. PMID: 10464374
-
Environmental novelty differentially affects c-fos mRNA expression induced by amphetamine or cocaine in subregions of the bed nucleus of the stria terminalis and amygdala.J Neurosci. 2001 Jan 15;21(2):732-40. doi: 10.1523/JNEUROSCI.21-02-00732.2001. J Neurosci. 2001. PMID: 11160452 Free PMC article.
-
Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis.J Neurosci Res. 2012 Jan;90(1):179-92. doi: 10.1002/jnr.22737. Epub 2011 Sep 15. J Neurosci Res. 2012. PMID: 21922520
-
Region-specific immediate-early gene expression following the administration of corticotropin-releasing hormone in virgin and lactating rats.Brain Res. 1997 Oct 3;770(1-2):151-62. doi: 10.1016/s0006-8993(97)00764-6. Brain Res. 1997. PMID: 9372214
Cited by
-
Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats.J Neuroendocrinol. 2010 Aug;22(8):872-88. doi: 10.1111/j.1365-2826.2010.02007.x. Epub 2010 Apr 16. J Neuroendocrinol. 2010. PMID: 20406350 Free PMC article.
-
Evidence for the Integration of Stress-Related Signals by the Rostral Posterior Hypothalamic Nucleus in the Regulation of Acute and Repeated Stress-Evoked Hypothalamo-Pituitary-Adrenal Response in Rat.J Neurosci. 2016 Jan 20;36(3):795-805. doi: 10.1523/JNEUROSCI.3413-15.2016. J Neurosci. 2016. PMID: 26791210 Free PMC article.
-
Corticotropin-Releasing Factor (CRF) and Addictive Behaviors.Int Rev Neurobiol. 2017;136:5-51. doi: 10.1016/bs.irn.2017.06.004. Epub 2017 Aug 7. Int Rev Neurobiol. 2017. PMID: 29056155 Free PMC article. Review.
-
Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas, but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone.Psychopharmacology (Berl). 2006 Apr;185(2):188-200. doi: 10.1007/s00213-005-0301-3. Epub 2006 Feb 10. Psychopharmacology (Berl). 2006. PMID: 16470400
-
Sensorimotor modulation of mood and depression: in search of an optimal mode of stimulation.Front Hum Neurosci. 2013 Jul 30;7:428. doi: 10.3389/fnhum.2013.00428. eCollection 2013. Front Hum Neurosci. 2013. PMID: 23908624 Free PMC article.
References
-
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 495–578.
-
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. - PubMed
-
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

