Surface expression, single-channel analysis and membrane topology of recombinant Chlamydia trachomatis Major Outer Membrane Protein
- PMID: 15673471
- PMCID: PMC549562
- DOI: 10.1186/1471-2180-5-5
Surface expression, single-channel analysis and membrane topology of recombinant Chlamydia trachomatis Major Outer Membrane Protein
Abstract
Background: Chlamydial bacteria are obligate intracellular pathogens containing a cysteine-rich porin (Major Outer Membrane Protein, MOMP) with important structural and, in many species, immunity-related roles. MOMP forms extensive disulphide bonds with other chlamydial proteins, and is difficult to purify. Leaderless, recombinant MOMPs expressed in E. coli have yet to be refolded from inclusion bodies, and although leadered MOMP can be expressed in E. coli cells, it often misfolds and aggregates. We aimed to improve the surface expression of correctly folded MOMP to investigate the membrane topology of the protein, and provide a system to display native and modified MOMP epitopes.
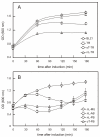
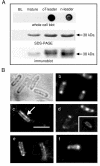
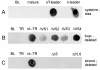
Results: C. trachomatis MOMP was expressed on the surface of E. coli cells (including "porin knockout" cells) after optimizing leader sequence, temperature and medium composition, and the protein was functionally reconstituted at the single-channel level to confirm it was folded correctly. Recombinant MOMP formed oligomers even in the absence of its 9 cysteine residues, and the unmodified protein also formed inter- and intra-subunit disulphide bonds. Its topology was modeled as a (16-stranded) beta-barrel, and specific structural predictions were tested by removing each of the four putative surface-exposed loops corresponding to highly immunogenic variable sequence (VS) domains, and one or two of the putative transmembrane strands. The deletion of predicted external loops did not prevent folding and incorporation of MOMP into the E. coli outer membrane, in contrast to the removal of predicted transmembrane strands.
Conclusions: C. trachomatis MOMP was functionally expressed on the surface of E. coli cells under newly optimized conditions. Tests of its predicted membrane topology were consistent with beta-barrel oligomers in which major immunogenic regions are displayed on surface-exposed loops. Functional surface expression, coupled with improved understanding of MOMP's topology, could provide modified antigens for immunological studies and vaccination, including live subunit vaccines, and might be useful to co-express MOMP with other chlamydial membrane proteins.
Figures

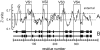




Similar articles
-
Recombinant expression of Chlamydia trachomatis major outer membrane protein in E. Coli outer membrane as a substrate for vaccine research.BMC Microbiol. 2016 Jul 27;16(1):165. doi: 10.1186/s12866-016-0787-3. BMC Microbiol. 2016. PMID: 27464881 Free PMC article.
-
Mutagenesis and functional reconstitution of chlamydial major outer membrane proteins: VS4 domains are not required for pore formation but modify channel function.Infect Immun. 2001 Mar;69(3):1671-8. doi: 10.1128/IAI.69.3.1671-1678.2001. Infect Immun. 2001. PMID: 11179342 Free PMC article.
-
Prediction of the membrane-spanning beta-strands of the major outer membrane protein of Chlamydia.Protein Sci. 2002 Jul;11(7):1854-61. doi: 10.1110/ps.3650102. Protein Sci. 2002. PMID: 12070338 Free PMC article.
-
Chlamydia trachomatis antigens: role in immunity and pathogenesis.Infect Agents Dis. 1994 Oct;3(5):218-33. Infect Agents Dis. 1994. PMID: 7866655 Review.
-
Advances in Chlamydia trachomatis Vaccination: Unveiling the Potential of Major Outer Membrane Protein Derivative Constructs.Microorganisms. 2024 Jun 13;12(6):1196. doi: 10.3390/microorganisms12061196. Microorganisms. 2024. PMID: 38930578 Free PMC article. Review.
Cited by
-
Recombinant expression of Chlamydia trachomatis major outer membrane protein in E. Coli outer membrane as a substrate for vaccine research.BMC Microbiol. 2016 Jul 27;16(1):165. doi: 10.1186/s12866-016-0787-3. BMC Microbiol. 2016. PMID: 27464881 Free PMC article.
-
A novel transport mechanism for MOMP in Chlamydophila pneumoniae and its putative role in immune-therapy.PLoS One. 2013 Apr 24;8(4):e61139. doi: 10.1371/journal.pone.0061139. Print 2013. PLoS One. 2013. PMID: 23637791 Free PMC article.
-
Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis.J Bacteriol. 2007 Sep;189(17):6222-35. doi: 10.1128/JB.00552-07. Epub 2007 Jun 29. J Bacteriol. 2007. PMID: 17601785 Free PMC article.
-
A 3-dimensional trimeric β-barrel model for Chlamydia MOMP contains conserved and novel elements of Gram-negative bacterial porins.PLoS One. 2013 Jul 25;8(7):e68934. doi: 10.1371/journal.pone.0068934. Print 2013. PLoS One. 2013. PMID: 23935908 Free PMC article.
-
Super-Resolution Fluorescence Microscopy Reveals Clustering Behaviour of Chlamydia pneumoniae's Major Outer Membrane Protein.Biology (Basel). 2020 Oct 20;9(10):344. doi: 10.3390/biology9100344. Biology (Basel). 2020. PMID: 33092039 Free PMC article.
References
-
- Stephens ES, ed . Chlamydia intracellular biology, pathogenesis, and immunity. ASM Press, Washington, DC; 1999.
-
- Salari SH, Ward ME. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981;123:197–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

