Identification of xenopus CENP-A and an associated centromeric DNA repeat
- PMID: 15673610
- PMCID: PMC1073662
- DOI: 10.1091/mbc.e04-09-0788
Identification of xenopus CENP-A and an associated centromeric DNA repeat
Abstract
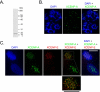
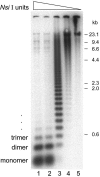
Kinetochores are the proteinaceous complexes that assemble on centromeric DNA and direct eukaryotic chromosome segregation. The mechanisms by which higher eukaryotic cells define centromeres are poorly understood. Possible molecular contributors to centromere specification include the underlying DNA sequences and epigenetic factors such as binding of the centromeric histone centromere protein A (CENP-A). Frog egg extracts are an attractive system for studying centromere definition and kinetochore assembly. To facilitate such studies, we cloned a Xenopus laevis homologue of CENP-A (XCENP-A). We identified centromere-associated DNA sequences by cloning fragments of DNA that copurified with XCENP-A by chromatin immunoprecipitation. XCENP-A associates with frog centromeric repeat 1 (Fcr1), a 174-base pair repeat containing a possible CENP-B box. Southern blots of partially digested genomic DNA revealed large ordered arrays of Fcr1 in the genome. Fluorescent in situ hybridization with Fcr1 probes stained most centromeres in cultured cells. By staining lampbrush chromosomes, we specifically identified the 11 (of 18) chromosomes that stain consistently with Fcr1 probes.
Figures




References
-
- Alonso, A., Mahmood, R., Li, S., Cheung, F., Yoda, K., and Warburton, P. E. (2003). Genomic microarray analysis reveals distinct locations for the CENP-A binding domains in three human chromosome 13q32 neocentromeres. Hum. Mol. Genet. 12, 2711–2721. - PubMed
-
- Biggins, S., and Walczak, C. E. (2003). Captivating capture: how microtubules attach to kinetochores. Curr. Biol. 13, R449–R460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

