Determinants of the endosomal localization of sorting nexin 1
- PMID: 15673616
- PMCID: PMC1073682
- DOI: 10.1091/mbc.e04-06-0504
Determinants of the endosomal localization of sorting nexin 1
Abstract
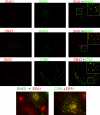
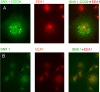
The sorting nexin (SNX) family of proteins is characterized by sequence-related phox homology (PX) domains. A minority of PX domains bind with high affinity to phosphatidylinositol 3-phosphate [PI(3)P], whereas the majority of PX domains exhibit low affinity that is insufficient to target them to vesicles. SNX1 is located on endosomes, but its low affinity PX domain fails to localize in vivo. The NMR structure of the PX domain of SNX1 reveals an overall fold that is similar to high-affinity PX domains. However, the phosphatidylinositol (PI) binding pocket of the SNX1 PX domain is incomplete; regions of the pocket that are well defined in high-affinity PX domains are highly mobile in SNX1. Some of this mobility is lost upon binding PI(3)P. The C-terminal domain of SNX1 is a long helical dimer that localizes to vesicles but not to the early endosome antigen-1-containing vesicles where endogenous SNX1 resides. Thus, the obligate dimerization of SNX1 that is driven by the C-terminal domain creates a high-affinity PI binding species that properly targets the holo protein to endosomes.
Figures
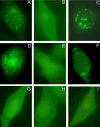
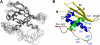
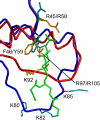
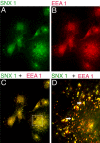

Similar articles
-
Retromer.Curr Opin Cell Biol. 2008 Aug;20(4):427-36. doi: 10.1016/j.ceb.2008.03.009. Epub 2008 May 9. Curr Opin Cell Biol. 2008. PMID: 18472259 Free PMC article. Review.
-
Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides.Curr Biol. 2004 Oct 26;14(20):1791-800. doi: 10.1016/j.cub.2004.09.077. Curr Biol. 2004. PMID: 15498486
-
A large family of endosome-localized proteins related to sorting nexin 1.Biochem J. 2001 Aug 15;358(Pt 1):7-16. doi: 10.1042/0264-6021:3580007. Biochem J. 2001. PMID: 11485546 Free PMC article.
-
Endosomal localization and function of sorting nexin 1.Proc Natl Acad Sci U S A. 2002 May 14;99(10):6767-72. doi: 10.1073/pnas.092142699. Epub 2002 May 7. Proc Natl Acad Sci U S A. 2002. PMID: 11997453 Free PMC article.
-
The Phox (PX) domain proteins and membrane traffic.Biochim Biophys Acta. 2006 Aug;1761(8):878-96. doi: 10.1016/j.bbalip.2006.04.011. Epub 2006 May 6. Biochim Biophys Acta. 2006. PMID: 16782399 Review.
Cited by
-
A novel live-cell imaging assay reveals regulation of endosome maturation.Elife. 2021 Nov 30;10:e70982. doi: 10.7554/eLife.70982. Elife. 2021. PMID: 34846303 Free PMC article.
-
Clathrin is not required for SNX-BAR-retromer-mediated carrier formation.J Cell Sci. 2013 Jan 1;126(Pt 1):45-52. doi: 10.1242/jcs.112904. Epub 2012 Sep 26. J Cell Sci. 2013. PMID: 23015596 Free PMC article.
-
Mechanistic similarities in docking of the FYVE and PX domains to phosphatidylinositol 3-phosphate containing membranes.Prog Lipid Res. 2007 Nov;46(6):315-27. doi: 10.1016/j.plipres.2007.06.001. Epub 2007 Jul 13. Prog Lipid Res. 2007. PMID: 17707914 Free PMC article. Review.
-
Retromer.Curr Opin Cell Biol. 2008 Aug;20(4):427-36. doi: 10.1016/j.ceb.2008.03.009. Epub 2008 May 9. Curr Opin Cell Biol. 2008. PMID: 18472259 Free PMC article. Review.
-
Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors.Mol Cell Biol. 2007 Feb;27(3):1112-24. doi: 10.1128/MCB.00156-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101778 Free PMC article.
References
-
- Barr, V. A., Phillips, S. A., Taylor, S. I., and Haft, C. R. (2000). Overexpression of a novel sorting Nexin, SNX15, affects endosome morphology and protein trafficking. Traffic 1, 904-916. - PubMed
-
- Bravo, J., et al. (2001). The crystal structure of the PX domain from p40phox bound to phosphatidylinositol 3-phosphate. Mol. Cell 8, 829-839. - PubMed
-
- Brunger, A. T., et al. (1998). Crystallography and NMR System. Acta Crystallogr. D54, 905-921. - PubMed
-
- Burden, J. J., Sun, X.-M., Garcia, A. B. G., and Soutar, A. K. (2004). Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting Nexin 17. J. Biol. Chem. 279, 16237-16245. - PubMed
-
- Corlinescu, G., Delaglio, F., and Bax, A. (1999). Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR 13, 289-302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

