Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons
- PMID: 15673657
- PMCID: PMC6725618
- DOI: 10.1523/JNEUROSCI.4235-04.2005
Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons
Erratum in
- J Neurosci. 2010 Nov 10;30(45):np. Smit, August [corrected to Smit, August B]
Abstract
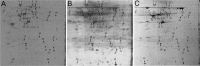
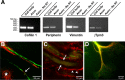
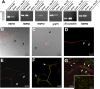
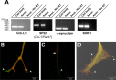
Recent studies have begun to focus on the signals that regulate axonal protein synthesis and the functional significance of localized protein synthesis. However, identification of proteins that are synthesized in mammalian axons has been mainly based on predictions. Here, we used axons purified from cultures of injury-conditioned adult dorsal root ganglion (DRG) neurons and proteomics methodology to identify axonally synthesized proteins. Reverse transcription (RT)-PCR from axonal preparations was used to confirm that the mRNA for each identified protein extended into the DRG axons. Proteins and the encoding mRNAs for the cytoskeletal proteins beta-actin, peripherin, vimentin, gamma-tropomyosin 3, and cofilin 1 were present in the axonal preparations. In addition to the cytoskeletal elements, several heat shock proteins (HSP27, HSP60, HSP70, grp75, alphaB crystallin), resident endoplasmic reticulum (ER) proteins (calreticulin, grp78/BiP, ERp29), proteins associated with neurodegenerative diseases (ubiquitin C-terminal hydrolase L1, rat ortholog of human DJ-1/Park7, gamma-synuclein, superoxide dismutase 1), anti-oxidant proteins (peroxiredoxins 1 and 6), and metabolic proteins (e.g., phosphoglycerate kinase 1 (PGK 1), alpha enolase, aldolase C/Zebrin II) were included among the axonally synthesized proteins. Detection of the mRNAs encoding each of the axonally synthesized proteins identified by mass spectrometry in the axonal compartment indicates that the DRG axons have the potential to synthesize a complex population of proteins. Local treatment of the DRG axons with NGF or BDNF increased levels of cytoskeletal mRNAs into the axonal compartment by twofold to fivefold but had no effect on levels of the other axonal mRNAs studied. Neurotrophins selectively increased transport of beta-actin, peripherin, and vimentin mRNAs from the cell body into the axons rather than changing transcription or mRNA survival in the axonal compartment.
Figures




Similar articles
-
A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons.J Neurosci. 2001 Dec 1;21(23):9291-303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. J Neurosci. 2001. PMID: 11717363 Free PMC article.
-
Sensory neurons selectively upregulate synthesis and transport of the beta III-tubulin protein during axonal regeneration.J Neurosci. 1995 Feb;15(2):1545-55. doi: 10.1523/JNEUROSCI.15-02-01545.1995. J Neurosci. 1995. PMID: 7869117 Free PMC article.
-
mRNAs and Protein Synthetic Machinery Localize into Regenerating Spinal Cord Axons When They Are Provided a Substrate That Supports Growth.J Neurosci. 2015 Jul 15;35(28):10357-70. doi: 10.1523/JNEUROSCI.1249-15.2015. J Neurosci. 2015. PMID: 26180210 Free PMC article.
-
Changes in cytoskeletal protein synthesis following axon injury and during axon regeneration.Mol Neurobiol. 1992 Summer-Fall;6(2-3):107-23. doi: 10.1007/BF02780547. Mol Neurobiol. 1992. PMID: 1476674 Review.
-
Translating regeneration: Local protein synthesis in the neuronal injury response.Neurosci Res. 2019 Feb;139:26-36. doi: 10.1016/j.neures.2018.10.003. Epub 2018 Oct 12. Neurosci Res. 2019. PMID: 30321567 Review.
Cited by
-
The top 100 most cited articles on axon regeneration from 2003 to 2023: a bibliometric analysis.Front Neurosci. 2024 Jun 26;18:1410988. doi: 10.3389/fnins.2024.1410988. eCollection 2024. Front Neurosci. 2024. PMID: 38988773 Free PMC article. Review.
-
Local synthesis of actin-binding protein beta-thymosin regulates neurite outgrowth.J Neurosci. 2006 Jan 4;26(1):152-7. doi: 10.1523/JNEUROSCI.4164-05.2006. J Neurosci. 2006. PMID: 16399682 Free PMC article.
-
Endogenous toll-like receptor ligands and their biological significance.J Cell Mol Med. 2010 Nov;14(11):2592-603. doi: 10.1111/j.1582-4934.2010.01127.x. J Cell Mol Med. 2010. PMID: 20629986 Free PMC article. Review.
-
The moonlighting protein c-Fos activates lipid synthesis in neurons, an activity that is critical for cellular differentiation and cortical development.J Biol Chem. 2020 Jun 26;295(26):8808-8818. doi: 10.1074/jbc.RA119.010129. Epub 2020 May 8. J Biol Chem. 2020. PMID: 32385110 Free PMC article.
-
Phase separation and pathologic transitions of RNP condensates in neurons: implications for amyotrophic lateral sclerosis, frontotemporal dementia and other neurodegenerative disorders.Front Mol Neurosci. 2023 Sep 1;16:1242925. doi: 10.3389/fnmol.2023.1242925. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37720552 Free PMC article. Review.
References
-
- Akbar M, Lundberg A, Liu K, Vidyadaran S, Wells K, Dolatshad H, Wynn S, Wells D, Latchman D, de Belleroche J (2003) The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem 278: 19956-19965. - PubMed
-
- Bamburg JR (1999) Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15: 185-230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
