The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein
- PMID: 15673660
- PMCID: PMC6725623
- DOI: 10.1523/JNEUROSCI.4464-04.2005
The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein
Abstract
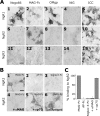
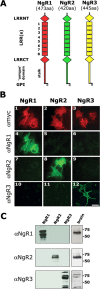
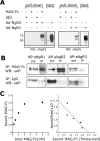
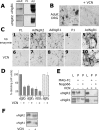
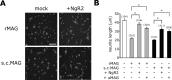
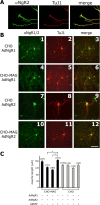
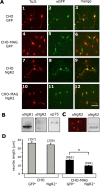
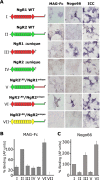
The Nogo-66 receptor (NgR1) is a promiscuous receptor for the myelin inhibitory proteins Nogo/Nogo-66, myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp). NgR1, an axonal glycoprotein, is the founding member of a protein family composed of the structurally related molecules NgR1, NgR2, and NgR3. Here we show that NgR2 is a novel receptor for MAG and acts selectively to mediate MAG inhibitory responses. MAG binds NgR2 directly and with greater affinity than NgR1. In neurons NgR1 and NgR2 support MAG binding in a sialic acid-dependent Vibrio cholerae neuraminidase-sensitive manner. Forced expression of NgR2 is sufficient to impart MAG inhibition to neonatal sensory neurons. Soluble NgR2 has MAG antagonistic capacity and promotes neuronal growth on MAG and CNS myelin substrate in vitro. Structural studies have revealed that the NgR2 leucine-rich repeat cluster and the NgR2 "unique" domain are necessary for high-affinity MAG binding. Consistent with its role as a neuronal MAG receptor, NgR2 is an axonassociated glycoprotein. In postnatal brain NgR1 and NgR2 are strongly enriched in Triton X-100-insoluble lipid rafts. Neural expression studies of NgR1 and NgR2 have revealed broad and overlapping, yet distinct, distribution in the mature CNS. Taken together, our studies identify NgRs as a family of receptors (or components of receptors) for myelin inhibitors and provide insights into how interactions between MAG and members of the Nogo receptor family function to coordinate myelin inhibitory responses.
Figures
References
-
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M (1995) Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 15: 1375-1381. - PubMed
-
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT (1999) Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22: 89-101. - PubMed
-
- Carim-Todd L, Escarceller M, Estivill X, Sumoy L (2003) LRRN6A/LERN1 (leucine-rich repeat neuronal protein 1), a novel gene with enriched expression in limbic system and neocortex. Eur J Neurosci 18: 3167-3182. - PubMed
-
- Chivatakarn O, Venkatesh K, Lee H, Giger RJ (2004) The pan-neurotrophin receptor p75NTR is not necessary for MAG inhibition. Soc Neurosci Abstr 30: 942.9.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
