The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain
- PMID: 15673661
- PMCID: PMC6725614
- DOI: 10.1523/JNEUROSCI.4335-04.2005
The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain
Abstract
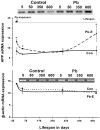
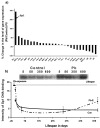
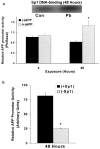
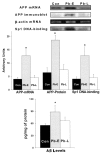
The fetal basis of adult disease (FeBAD) hypothesis states that many adult diseases have a fetal origin. According to FeBAD, injury or environmental influences occurring at critical periods of organ development could result in "programmatic" changes via alterations in gene expression or gene imprinting that may result in functional deficits that become apparent later in life. Alzheimer's disease (AD) is a progressive neurodegenerative disorder that is characterized by excessive deposits of aggregated beta-amyloid (Abeta) peptides, which are snippets of the beta-amyloid precursor protein (APP). The predominantly sporadic nature of AD suggests that the environment must play a role in neurodegeneration. To examine latent responses to an environmental agent, we exposed rodents to lead and monitored the lifetime expression of the APP gene. We observed that APP mRNA expression was transiently induced in neonates, but exhibited a delayed overexpression 20 months after exposure to Pb had ceased. This upregulation in APP mRNA expression was commensurate with a rise in activity of the transcription factor Sp1, one of the regulators of the APP gene. Furthermore, the increase in APP gene expression in old age was accompanied by an elevation in APP and its amyloidogenic Abeta product. In contrast, APP expression, Sp1 activity, as well as APP and Abeta protein levels were unresponsive to Pb exposure during old age. These data suggested that environmental influences occurring during brain development predetermined the expression and regulation of APP later in life, potentially altering the course of amyloidogenesis.
Figures
References
-
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI (1998) Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem 273: 12817-12826. - PubMed
-
- Bakheet SA, Zawia NH (2004) The role of pou domain transcription factors in lead neurotoxicity. In: Molecular neurotoxicology, environmental agents, and transcription-transduction coupling (Zawia NH, ed), pp 183-198. Boca Raton, FL: CRC.
-
- Barker DJ (2002) Fetal programming of coronary heart disease. Trends Endocrinol Metab 13: 364-368. - PubMed
-
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ (1989) Weight in infancy and death from ischaemic heart disease. Lancet 2: 577-580. - PubMed
-
- Barone Jr S, Stanton ME, Mundy WR (1995) Neurotoxic effects of neonatal triethyltin (TET) exposure are exacerbated with aging. Neurobiol Aging 16: 723-735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
