Mouse taste buds use serotonin as a neurotransmitter
- PMID: 15673664
- PMCID: PMC6725637
- DOI: 10.1523/JNEUROSCI.4446-04.2005
Mouse taste buds use serotonin as a neurotransmitter
Erratum in
- J Neurosci. 2015 Jun 10;35(23):8970
Abstract
Synapses between gustatory receptor cells and primary sensory afferent fibers transmit the output signal from taste buds to the CNS. Several transmitter candidates have been proposed for these synapses, including serotonin (5-HT), glutamate, acetylcholine, ATP, peptides, and others, but, to date, none has been unambiguously identified. We used Chinese hamster ovary cells stably expressing 5-HT2C receptors as biodetectors to monitor 5-HT release from taste buds. When taste buds were depolarized with KCl or stimulated with bitter, sweet, or sour (acid) tastants, serotonin was released. KCl- and acid-induced 5-HT release, but not release attributable to sweet or bitter stimulation, required Ca2+ influx. In contrast, 5-HT release evoked by sweet and bitter stimulation seemed to be triggered by intracellular Ca2+ release. These experiments strongly implicate serotonin as a taste bud neurotransmitter and reveal unexpected transmitter release mechanisms.
Figures
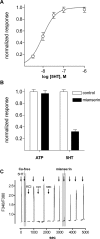
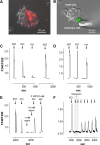

Similar articles
-
Signal transduction and information processing in mammalian taste buds.Pflugers Arch. 2007 Aug;454(5):759-76. doi: 10.1007/s00424-007-0247-x. Epub 2007 Apr 28. Pflugers Arch. 2007. PMID: 17468883 Free PMC article. Review.
-
Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells.PLoS One. 2011;6(10):e25471. doi: 10.1371/journal.pone.0025471. Epub 2011 Oct 20. PLoS One. 2011. PMID: 22028776 Free PMC article.
-
Using biosensors to detect the release of serotonin from taste buds during taste stimulation.Arch Ital Biol. 2005 May;143(2):87-96. Arch Ital Biol. 2005. PMID: 16106989 Free PMC article.
-
Calcitonin Gene-Related Peptide Reduces Taste-Evoked ATP Secretion from Mouse Taste Buds.J Neurosci. 2015 Sep 16;35(37):12714-24. doi: 10.1523/JNEUROSCI.0100-15.2015. J Neurosci. 2015. PMID: 26377461 Free PMC article.
-
Taste buds as peripheral chemosensory processors.Semin Cell Dev Biol. 2013 Jan;24(1):71-9. doi: 10.1016/j.semcdb.2012.12.002. Epub 2012 Dec 20. Semin Cell Dev Biol. 2013. PMID: 23261954 Free PMC article. Review.
Cited by
-
Expression profile of the zinc transporter ZnT3 in taste cells of rat circumvallate papillae and its role in zinc release, a potential mechanism for taste stimulation.Eur J Histochem. 2022 Nov 10;66(4):3534. doi: 10.4081/ejh.2022.3534. Eur J Histochem. 2022. PMID: 36373349 Free PMC article.
-
Immunocytochemical analysis of P2X2 in rat circumvallate taste buds.BMC Neurosci. 2012 May 23;13:51. doi: 10.1186/1471-2202-13-51. BMC Neurosci. 2012. PMID: 22621423 Free PMC article.
-
Signal transduction and information processing in mammalian taste buds.Pflugers Arch. 2007 Aug;454(5):759-76. doi: 10.1007/s00424-007-0247-x. Epub 2007 Apr 28. Pflugers Arch. 2007. PMID: 17468883 Free PMC article. Review.
-
The Functional and Neurobiological Properties of Bad Taste.Physiol Rev. 2019 Jan 1;99(1):605-663. doi: 10.1152/physrev.00044.2017. Physiol Rev. 2019. PMID: 30475657 Free PMC article. Review.
-
Taste bud cells of adult mice are responsive to Wnt/β-catenin signaling: implications for the renewal of mature taste cells.Genesis. 2011 Apr;49(4):295-306. doi: 10.1002/dvg.20731. Epub 2011 Apr 1. Genesis. 2011. PMID: 21328519 Free PMC article.
References
-
- Akiyoshi J, Nishizono A, Yamada K, Nagayama H, Mifune K, Fujii I (1995) Rapid desensitization of serotonin 5-HT2C receptor-stimulated intracellular calcium mobilization in CHO cells transfected with cloned human 5-HT2C receptors. J Neurochem 64: 2473-2479. - PubMed
-
- Akiyoshi J, Isogawa K, Yamada K, Nagayama H, Fujii I (1996) Effects of antidepressants on intracellular Ca2+ mobilization in CHO cells transfected with the human 5-HT2C receptors. Biol Psychiatry 39: 1000-1008. - PubMed
-
- Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S (1994) Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharmacol 46: 477-484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
