A novel substrate of receptor tyrosine phosphatase PTPRO is required for nerve growth factor-induced process outgrowth
- PMID: 15673668
- PMCID: PMC6725615
- DOI: 10.1523/JNEUROSCI.4365-04.2005
A novel substrate of receptor tyrosine phosphatase PTPRO is required for nerve growth factor-induced process outgrowth
Abstract
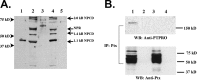
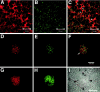
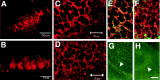
The receptor protein tyrosine phosphatase PTPRO may be involved in axon guidance both as a ligand and as a neuronal receptor. We have begun to characterize signaling by PTPRO as a receptor by screening for proteins interacting with the intracellular domain of PTPRO. In a yeast-two hybrid screen, we identified a novel class of protein, which we named neuronal pentraxin with chromo domain (NPCD), as a PTPRO-interacting protein. We have shown recently that NPCD has multiple cytoplasmic isoforms as a result of alternative splicing and that these proteins are present in many neurons, mainly associated with the inner side of the plasma membrane. Through additional two-hybrid experiments, cotransfection and reciprocal coprecipitation, glutathione S-transferase pulldown, and immunoprecipitation in vivo, we confirm that NPCD isoforms interact with the catalytic phosphatase domain of PTPRO. We also find that at least one NPCD isoform is tyrosine phosphorylated in vivo and can serve as a substrate for PTPRO in vitro. Analysis of PTPRO knock-out mice demonstrates that normal localization of NPCD at the plasma membrane requires PTPRO expression, suggesting a physiological role for the NPCD/PTPRO interaction. NPCD is likely to be relevant to axon growth and/or guidance, because RNA interference mediated knock-down of NPCD expression in pheochromocytoma cells inhibits NGF-induced neuronal process outgrowth without affecting NGF-dependent survival or initial NGF signaling.
Figures
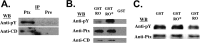
Similar articles
-
Neuronal pentraxin with chromo domain (NPCD) is a novel class of protein expressed in multiple neuronal domains.J Comp Neurol. 2005 Jan 24;481(4):391-402. doi: 10.1002/cne.20391. J Comp Neurol. 2005. PMID: 15593341
-
Identification of an ectodomain within the LAR protein tyrosine phosphatase receptor that binds homophilically and activates signalling pathways promoting neurite outgrowth.Eur J Neurosci. 2005 Nov;22(9):2159-70. doi: 10.1111/j.1460-9568.2005.04403.x. Eur J Neurosci. 2005. PMID: 16262654
-
Expression of PTPRO during mouse development suggests involvement in axonogenesis and differentiation of NT-3 and NGF-dependent neurons.J Comp Neurol. 2003 Feb 17;456(4):384-95. doi: 10.1002/cne.10532. J Comp Neurol. 2003. PMID: 12532410
-
Regulatory Functions of Protein Tyrosine Phosphatase Receptor Type O in Immune Cells.Front Immunol. 2021 Nov 22;12:783370. doi: 10.3389/fimmu.2021.783370. eCollection 2021. Front Immunol. 2021. PMID: 34880876 Free PMC article. Review.
-
GAP-43--what does it do in the growth cone?Trends Neurosci. 1989 Oct;12(10):363-5. doi: 10.1016/0166-2236(89)90073-8. Trends Neurosci. 1989. PMID: 2479132 Review. No abstract available.
Cited by
-
Antibodies to protein tyrosine phosphatase receptor type O (PTPro) increase glomerular albumin permeability (P(alb)).Am J Physiol Renal Physiol. 2009 Jul;297(1):F138-44. doi: 10.1152/ajprenal.00122.2008. Epub 2009 Apr 29. Am J Physiol Renal Physiol. 2009. PMID: 19403647 Free PMC article.
-
Protein tyrosine phosphatase PTPRO represses lung adenocarcinoma progression by inducing mitochondria-dependent apoptosis and restraining tumor metastasis.Cell Death Dis. 2024 Jan 5;15(1):11. doi: 10.1038/s41419-023-06375-x. Cell Death Dis. 2024. PMID: 38182570 Free PMC article.
-
Gene expression profiles of HIV-1-infected glia and brain: toward better understanding of the role of astrocytes in HIV-1-associated neurocognitive disorders.J Neuroimmune Pharmacol. 2010 Mar;5(1):44-62. doi: 10.1007/s11481-009-9167-1. Epub 2009 Aug 21. J Neuroimmune Pharmacol. 2010. PMID: 19697136 Free PMC article. Review.
-
Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-beta and is overexpressed in dystrophic neurites in Alzheimer's brain.J Neurosci. 2006 Dec 6;26(49):12735-47. doi: 10.1523/JNEUROSCI.0575-06.2006. J Neurosci. 2006. PMID: 17151277 Free PMC article.
-
Neuronal pentraxin 1 negatively regulates excitatory synapse density and synaptic plasticity.J Neurosci. 2015 Apr 8;35(14):5504-21. doi: 10.1523/JNEUROSCI.2548-14.2015. J Neurosci. 2015. PMID: 25855168 Free PMC article.
References
-
- Ashcroft M, Stephens RM, Hallberg B, Downward J, Kaplan DR (1999) The selective and inducible activation of endogenous PI 3-kinase in PC12 cells results in efficient NGF-mediated survival but defective neurite out-growth. Oncogene 18: 4586-4597. - PubMed
-
- Beltran PJ, Bixby JL (2003) Receptor protein tyrosine phosphatases as mediators of cellular adhesion. Front Biosci 8: D87-D99. - PubMed
-
- Beltran PJ, Bixby JL, Masters BA (2003) Expression of PTPRO during mouse development suggests involvement in axonogenesis and differentiation of NT-3 and NGF-dependent neurons. J Comp Neurol 456: 384-395. - PubMed
-
- Bixby JL (2000) Receptor tyrosine phosphatases in axon growth and guidance. NeuroReport 11: R5-R10. - PubMed
-
- Bixby JL (2001) Ligands and signaling through receptor-type tyrosine phosphatases. IUBMB Life 51: 157-163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
