Target-specific regulation of synaptic amplitudes in the neocortex
- PMID: 15673684
- PMCID: PMC6725631
- DOI: 10.1523/JNEUROSCI.3951-04.2005
Target-specific regulation of synaptic amplitudes in the neocortex
Abstract
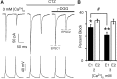
In layers 2/3 in the rat visual cortex, glutamatergic synapses, between pyramidal neurons and GABAergic interneurons, show target-specific depression or facilitation. To study the mechanisms regulating these short-term synaptic modifications, we recorded from synaptically connected pyramidal neurons (presynaptic) and multipolar or bitufted interneurons (postsynaptic). Evoked AMPA receptor (AMPAR)- or NMDA receptor (NMDAR)-mediated EPSCs were pharmacologically isolated at these pyramidal-to-interneuron synapses while altering release probability (P(r)) by changing the extracellular Ca2+ concentration ([Ca2+]o). At the pyramidal-to-multipolar synapse, which shows paired-pulse depression, elevation of [Ca2+]o from physiological concentrations (2 mm) to 3 mm increased the amplitude of the initial AMPAR-mediated EPSC and enhanced paired-pulse depression. In contrast, the initial NMDAR-mediated EPSC did not change in amplitude with raised P(r) nor was paired-pulse depression altered. This lack of an increase of NMDAR-mediated currents is not a result of Ca2+-dependent effects on the NMDAR. Rather, at the pyramidal-to-multipolar synapse, raised P(r) increases the transient glutamate concentration at individual release sites, possibly reflecting multivesicular release. In contrast, at the pyramidal-to-bitufted synapse, which shows facilitation, AMPAR- and NMDAR-meditated EPSCs showed parallel increases in response to raised P(r). Thus, our results reveal differential recruitment of AMPA and NMDARs at depressing and facilitating synapses in layers 2/3 of the cortex and suggest that the mechanisms regulating dynamic aspects of synaptic transmission are target specific.
Figures






Similar articles
-
Properties of excitatory synaptic connections mediated by the corpus callosum in the developing rat neocortex.J Neurophysiol. 2001 Dec;86(6):2973-85. doi: 10.1152/jn.2001.86.6.2973. J Neurophysiol. 2001. PMID: 11731554
-
Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics.J Physiol. 2001 Mar 15;531(Pt 3):807-26. doi: 10.1111/j.1469-7793.2001.0807h.x. J Physiol. 2001. PMID: 11251060 Free PMC article.
-
PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex.J Physiol. 2003 Feb 1;546(Pt 3):859-67. doi: 10.1113/jphysiol.2002.031369. J Physiol. 2003. PMID: 12563010 Free PMC article.
-
Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels.J Physiol. 2008 Mar 15;586(6):1475-80. doi: 10.1113/jphysiol.2007.148353. Epub 2007 Dec 20. J Physiol. 2008. PMID: 18096597 Free PMC article. Review.
-
TwoB or not twoB: differential transmission at glutamatergic mossy fiber-interneuron synapses in the hippocampus.Trends Neurosci. 2002 Dec;25(12):600-3. doi: 10.1016/s0166-2236(02)02259-2. Trends Neurosci. 2002. PMID: 12446120 Review.
Cited by
-
Lactate Attenuates Synaptic Transmission and Affects Brain Rhythms Featuring High Energy Expenditure.iScience. 2020 Jul 24;23(7):101316. doi: 10.1016/j.isci.2020.101316. Epub 2020 Jun 27. iScience. 2020. PMID: 32653807 Free PMC article.
-
Synapse-associated protein 97 regulates the membrane properties of fast-spiking parvalbumin interneurons in the visual cortex.J Neurosci. 2013 Jul 31;33(31):12739-50. doi: 10.1523/JNEUROSCI.0040-13.2013. J Neurosci. 2013. PMID: 23904610 Free PMC article.
-
Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing.J Neurosci. 2007 Feb 7;27(6):1325-33. doi: 10.1523/JNEUROSCI.2676-06.2007. J Neurosci. 2007. PMID: 17287507 Free PMC article.
-
Multivesicular release at Schaffer collateral-CA1 hippocampal synapses.J Neurosci. 2006 Jan 4;26(1):210-6. doi: 10.1523/JNEUROSCI.4307-05.2006. J Neurosci. 2006. PMID: 16399689 Free PMC article.
-
Target-cell-specific Short-term Plasticity Reduces the Excitatory Drive onto CA1 Interneurons Relative to Pyramidal Cells During Physiologically-derived Spike Trains.Neuroscience. 2018 Sep 15;388:430-447. doi: 10.1016/j.neuroscience.2018.07.051. Epub 2018 Aug 10. Neuroscience. 2018. PMID: 30099117 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
