Bayesian parameter estimates of nelfinavir and its active metabolite, hydroxy-tert-butylamide, in infants perinatally infected with human immunodeficiency virus type 1
- PMID: 15673728
- PMCID: PMC547202
- DOI: 10.1128/AAC.49.2.525-535.2005
Bayesian parameter estimates of nelfinavir and its active metabolite, hydroxy-tert-butylamide, in infants perinatally infected with human immunodeficiency virus type 1
Abstract
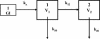
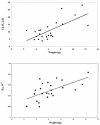
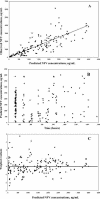
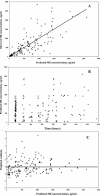
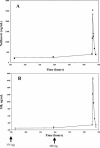
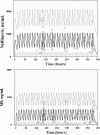
The objective of the present study was to develop a population pharmacokinetic model for nelfinavir mesylate (NFV) and nelfinavir hydroxy-tert-butylamide (M8), the most abundant metabolite of NFV, in infants vertically infected with human immunodeficiency virus type 1 and participating in the Paediatric European Network for Treatment of AIDS 7 study. Plasma NFV concentrations were determined during repeated NFV administrations (two to three times a day). Eighteen infants younger that age 2 years participated in this study. The doses administered ranged from 71 to 203 mg/kg of body weight/day. Pharmacokinetic parameter estimates were obtained by a compartmental approach by using a kinetic model to simultaneously fit NFV and M8 (active metabolite) concentrations. M8 was shown to be formation rate limited and was characterized by first-order rate constants of formation and elimination. Body weight was found to be a more appropriate predictor than age of the changes in (i) the rate of metabolism, (ii) the elimination rate constant of NFV, and (iii) NFV clearance. Population parameters were computed to account for the relationship between the rate of metabolism and body weight. The estimated NFV and M8 elimination half-lives were 4.3 and 2.04 h, respectively. The estimated NFV clearance was 2.13 liters/h/kg. The M8 concentration-to-NFV concentration ratio was 0.64 +/- 0.44. In conclusion, the population pharmacokinetic model describing the dispositions of NFV and M8 should facilitate the design of future studies to elucidate the relative contributions of the parent compound and M8 to the pharmacological and toxic effects of NFV therapy.
Figures
References
-
- Bardsley-Elliot, A., and G. L. Plosker. 2000. Nelfinavir, an update of its use in HIV infection. Drugs 59:581-620. - PubMed
-
- Beal, S. L., and L. B. Sheiner. 1994. NONMEM user's guide. University of California at San Francisco, San Francisco, Calif.
-
- Bergshoeff, A. S., P. L. Fraaij, A. M. van Rossum, T. F. Wolfs, S. P. Geelen, R. de Groot, and D. M. Burger. 2003. Pharmacokinetics of nelfinavir in children: influencing factors and dose implications. Antivir. Ther. 8:215-222. - PubMed
-
- Capparelli, E. V., J. L. Sullivan, L. Mofenson, E. Smith, B. Graham, P. Britto, M. I. Becker, D. Holland, J. D. Connor, and K. Luzuriaga. 2001. Pharmacokinetics of nelfinavir in human immunodeficiency virus-infected infants. Pediatr. Infect. Dis. J. 20:746-751. - PubMed
-
- Centers for Disease Control and Prevention. 1998. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Morb. Mortal. Wkly. Rep. 47:1-45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

