cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol
- PMID: 15673737
- PMCID: PMC547270
- DOI: 10.1128/AAC.49.2.584-589.2005
cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol
Abstract
Candida albicans biofilms are structured microbial communities with high levels of drug resistance. Farnesol, a quorum-sensing molecule that inhibits hyphal formation in C. albicans, has been found to prevent biofilm formation by C. albicans. There is limited information, however, about the molecular mechanism of farnesol against biofilm formation. We used cDNA microarray analysis to identify the changes in the gene expression profile of a C. albicans biofilm inhibited by farnesol. Confocal scanning laser microscopy was used to visualize and confirm normal and farnesol-inhibited biofilms. A total of 274 genes were identified as responsive, with 104 genes up-regulated and 170 genes down-regulated. Independent reverse transcription-PCR analysis was used to confirm the important changes detected by microarray analysis. In addition to hyphal formation-associated genes (e.g., TUP1, CRK1, and PDE2), a number of other genes with roles related to drug resistance (e.g., FCR1 and PDR16), cell wall maintenance (e.g., CHT2 and CHT3), and iron transport (e.g., FTR2) were responsive, as were several genes encoding heat shock proteins (e.g., HSP70, HSP90, HSP104, CaMSI3, and SSA2). Further study of these differentially regulated genes is warranted to evaluate how they may be involved in C. albicans biofilm formation. Consistent with the down-regulation of the cell surface hydrophobicity-associated gene (CSH1), the water-hydrocarbon two-phase assay showed a decrease in cell surface hydrophobicity in the farnesol-treated group compared to that in the control group. Our data provide new insight into the molecular mechanism of farnesol against C. albicans biofilm formation.
Figures


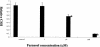
References
-
- Anaissie, E. J., J. H. Rex, O. Uzun, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. - PubMed
-
- Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105-109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

