Structure-activity relationships of different beta-lactam antibiotics against a soluble form of Enterococcus faecium PBP5, a type II bacterial transpeptidase
- PMID: 15673741
- PMCID: PMC547200
- DOI: 10.1128/AAC.49.2.612-618.2005
Structure-activity relationships of different beta-lactam antibiotics against a soluble form of Enterococcus faecium PBP5, a type II bacterial transpeptidase
Abstract
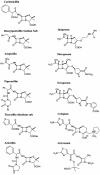
Penicillin-binding proteins (PBPs) catalyze the essential reactions in the biosynthesis of cell wall peptidoglycan from glycopeptide precursors. beta-Lactam antibiotics normally interfere with this process by reacting covalently with the active site serine to form a stable acyl-enzyme. The design of novel beta-lactams active against penicillin-susceptible and penicillin-resistant organisms will require a better understanding of the molecular details of this reaction. To that end, we compared the affinities of different beta-lactam antibiotics to a modified soluble form of a resistant Enterococcus faecium PBP5 (Delta1-36 rPBP5). The soluble protein, Delta1-36 rPBP5, was expressed in Escherichia coli and purified, and the NH(2)-terminal protein sequence was verified by amino acid sequencing. Using beta-lactams with different R1 side chains, we show that azlocillin has greater affinity for Delta1-36 rPBP5 than piperacillin and ampicillin (apparent K(i) = 7 +/- 0.3 microM, compared to 36 +/- 3 and 51 +/- 10 microM, respectively). Azlocillin also exhibits the most rapid acylation rate (apparent k(2) = 15 +/- 4 M(-1) s(-1)). Meropenem demonstrates an affinity for Delta1-36 rPBP5 comparable to that of ampicillin (apparent K(i) = 51 +/- 15 microM) but is slower at acylating (apparent k(2) = 0.14 +/- 0.02 M(-1) s(-1)). This characterization defines important structure-activity relationships for this clinically relevant type II transpeptidase, shows that the rate of formation of the acyl-enzyme is an essential factor determining the efficacy of a beta-lactam, and suggests that the specific side chain interactions of beta-lactams could be modified to improve inactivation of resistant PBPs.
Figures




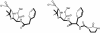
Similar articles
-
The structures of penicillin-binding protein 4 (PBP4) and PBP5 from Enterococci provide structural insights into β-lactam resistance.J Biol Chem. 2018 Nov 30;293(48):18574-18584. doi: 10.1074/jbc.RA118.006052. Epub 2018 Oct 24. J Biol Chem. 2018. PMID: 30355734 Free PMC article.
-
Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium.J Bacteriol. 1996 Aug;178(16):4948-57. doi: 10.1128/jb.178.16.4948-4957.1996. J Bacteriol. 1996. PMID: 8759860 Free PMC article.
-
Kinetic features of L,D-transpeptidase inactivation critical for β-lactam antibacterial activity.PLoS One. 2013 Jul 4;8(7):e67831. doi: 10.1371/journal.pone.0067831. Print 2013. PLoS One. 2013. PMID: 23861815 Free PMC article.
-
Intrinsic penicillin resistance in enterococci.Microb Drug Resist. 1996 Summer;2(2):209-13. doi: 10.1089/mdr.1996.2.209. Microb Drug Resist. 1996. PMID: 9158761 Review.
-
Breaking down the cell wall: Strategies for antibiotic discovery targeting bacterial transpeptidases.Eur J Med Chem. 2020 May 15;194:112262. doi: 10.1016/j.ejmech.2020.112262. Epub 2020 Mar 23. Eur J Med Chem. 2020. PMID: 32248005 Review.
Cited by
-
Synthesis of labeled meropenem for the analysis of M. tuberculosis transpeptidases.Tetrahedron Lett. 2010;51(1):197-200. doi: 10.1016/j.tetlet.2009.10.124. Tetrahedron Lett. 2010. PMID: 20161438 Free PMC article.
-
Molecular Features of Cephalosporins Important for Activity against Antimicrobial-Resistant Neisseria gonorrhoeae.ACS Infect Dis. 2021 Feb 12;7(2):293-308. doi: 10.1021/acsinfecdis.0c00400. Epub 2021 Feb 3. ACS Infect Dis. 2021. PMID: 33533239 Free PMC article.
-
The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition.Front Microbiol. 2022 Mar 24;13:807955. doi: 10.3389/fmicb.2022.807955. eCollection 2022. Front Microbiol. 2022. PMID: 35401470 Free PMC article. Review.
-
Structural and Regulatory Changes in PBP4 Trigger Decreased β-Lactam Susceptibility in Enterococcus faecalis.mBio. 2018 Apr 3;9(2):e00361-18. doi: 10.1128/mBio.00361-18. mBio. 2018. PMID: 29615500 Free PMC article.
-
L,D-Transpeptidase Specific Probe Reveals Spatial Activity of Peptidoglycan Cross-Linking.ACS Chem Biol. 2019 Oct 18;14(10):2185-2196. doi: 10.1021/acschembio.9b00427. Epub 2019 Sep 16. ACS Chem Biol. 2019. PMID: 31487148 Free PMC article.
References
-
- Frere, J. M., M. Nguyen-Disteche, J. Coyette, and B. Joris. 1992. Mode of action: interaction with the penicillin-binding proteins. Chapman and Hall, Glasgow, Scotland.
-
- Ghuysen, J. M. 1994. Molecular structures of penicillin-binding proteins and beta-lactamases. Trends Microbiol. 2:372-380. - PubMed
-
- Goffin, C., and J. M. Ghuysen. 2002. Biochemistry and comparative genomics of SxxK superfamily acyltransferases offer a clue to the mycobacterial paradox: presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent. Microbiol. Mol. Biol. Rev. 66:702-738. - PMC - PubMed
-
- Golemi-Kotra, D., J. Y. Cha, S. O. Meroueh, S. B. Vakulenko, and S. Mobashery. 2003. Resistance to β-lactam antibiotics and its mediation by the sensor domain of the transmembrane BlaR signaling pathway in Staphylococcus aureus. J. Biol. Chem. 278:18419-18425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

