Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia
- PMID: 15674344
- PMCID: PMC1361530
- DOI: 10.1038/sj.onc.1208307
Induction of KLF4 in basal keratinocytes blocks the proliferation-differentiation switch and initiates squamous epithelial dysplasia
Abstract
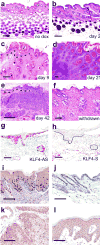
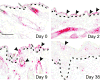
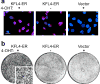
KLF4/GKLF normally functions in differentiating epithelial cells, but also acts as a transforming oncogene in vitro. To examine the role of this zinc finger protein in skin, we expressed the wild-type human allele from inducible and constitutive promoters. When induced in basal keratinocytes, KLF4 rapidly abolished the distinctive properties of basal and parabasal epithelial cells. KLF4 caused a transitory apoptotic response and the skin progressed through phases of hyperplasia and dysplasia. By 6 weeks, lesions exhibited nuclear KLF4 and other morphologic and molecular similarities to squamous cell carcinoma in situ. p53 determined the patch size sufficient to establish lesions, as induction in a mosaic pattern produced skin lesions only when p53 was deficient. Compared with p53 wild-type animals, p53 hemizygous animals had early onset of lesions and a pronounced fibrovascular response that included outgrowth of subcutaneous sarcoma. A KLF4-estrogen receptor fusion protein showed tamoxifen-dependent nuclear localization and conditional transformation in vitro. The results suggest that KLF4 can function in the nucleus to induce squamous epithelial dysplasia, and indicate roles for p53 and epithelial-mesenchymal signaling in these early neoplastic lesions.
Figures




References
-
- Adam PJ, Regan CP, Hautmann MB, Owens GK. J Biol Chem. 2000;275:37798–37806. - PubMed
-
- Boyle JO, Hakim J, Koch W, van der RP, Hruban RH, Roa RA, Correo R, Eby YJ, Ruppert JM, Sidransky D. Cancer Res. 1993;53:4477–4480. - PubMed
-
- Brash DE, Ponten J. Cancer Surv. 1998;32:69–113. - PubMed
-
- Brown K, Balmain A. Cancer Metastasis Rev. 1995;14:113–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

