Inhaled beclomethasone versus placebo for chronic asthma
- PMID: 15674896
- PMCID: PMC8447862
- DOI: 10.1002/14651858.CD002738.pub2
Inhaled beclomethasone versus placebo for chronic asthma
Abstract
Background: Inhaled beclomethasone dipropionate (BDP) has been, together with inhaled budesonide, the mainstay of anti-inflammatory therapy for asthma for many years. A range of new prophylactic therapies for asthma is becoming available and BDP has been reformulated using a hydrofluoroalkane-134a (HFA) propellant which is free from chlorofluorocarbon (CFC).
Objectives: The objectives of this review were to: (1) Compare the efficacy of BDP with placebo with both CFC and HFA propellants in the treatment of chronic asthma. (2) Explore the possibility that a dose response relationship exists for BDP in the treatment of chronic asthma. (3) To provide the best estimate of the efficacy of BDP as a benchmark for evaluation of newer asthma therapies.
Search strategy: Electronic searches were current as of January 2003.
Selection criteria: Randomised parallel group design trials for a minimum period of four weeks, in children and adults comparing CFC-BDP or HFA-BDP with placebo in the treatment of chronic asthma. Two reviewers independently assessed articles for inclusion and methodological quality.
Data collection and analysis: One reviewer extracted data; authors were contacted to clarify missing information. We analysed data with RevMan Analyses 1.0.2.
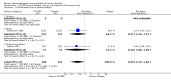
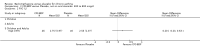
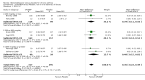
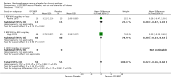
Main results: 60 studies recruiting 6542 participants met the inclusion criteria. CFC-BDP (57 studies): In non-oral steroid treated patients, at doses of 400 mcg/day or less CFC-BDP produced significant improvements from baseline in a number of efficacy measures compared with placebo, including forced expiratory volume in one second (FEV1) 360 ml (95% CI 260 to 460); FEV1 (% predicted) WMD 12.41% (95% CI 8.18 to 16.64) and morning peak expiratory flow rate (am PEF) WMD 35.95 L/min (95% CI 27.85 to 44.04). BDP also led to reductions in rescue beta-2 agonist use compared with placebo of -2.32 puffs/d (95% CI -2.55 to -2.09) and reduced the relative risk (RR) of trial withdrawal due to an asthma exacerbation 0.25 (95% CI 0.12 to 0.51). Subgroup analyses based on treatment duration provide support to the proposal that a treatment period of greater than four weeks is required to realise a fuller treatment effect. In oral steroid treated patients BDP led to significantly greater reductions in oral prednisolone use WMD -4.91 mg/d (95% CI -5.88 to -3.94 mg/d) and greater likelihood of withdrawing oral steroid treatment RR 8.02 (95% CI 3.23 to 19.92). HFA-BDP (3 studies): In non-oral steroid-treated patients, HFA-BDP was significantly more effective than placebo in improving FEV1, morning and evening PEF, FEF25 to 75%, reduced asthma symptoms and beta2-agonists daily consumption. Significant effects for such outcomes were apparent after six weeks of treatment. In oral steroid treated patients, HFA-BDP improved significantly FEV1 and am PEF. The summary estimates for these outcomes suggested a high level of heterogeneity, and divergent aims of the studies may contribute to the variation we observed. Limited data on adverse events were reported.
Authors' conclusions: This review has quantified the efficacy of CFC-BDP and HFA-BDP in the treatment of chronic asthma and strongly supports its use. Current asthma guidelines recommend titration of dose to individual patient response, but the published data provide little support for dose titration above 400 mcg/d in patients with mild to moderate asthma. There are insufficient data to draw any conclusions concerning dose-response in people with severe asthma.
Conflict of interest statement
None
Figures
























































































































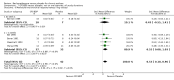










































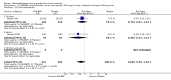




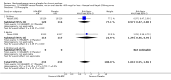




























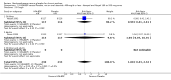


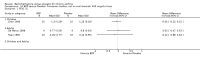




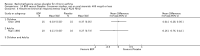







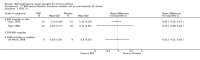
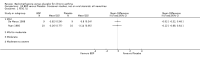
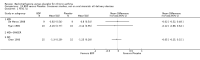
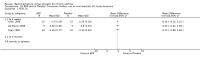
Update of
-
Inhaled beclomethasone versus placebo for chronic asthma.Cochrane Database Syst Rev. 2000;(4):CD002738. doi: 10.1002/14651858.CD002738. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2005 Jan 25;(1):CD002738. doi: 10.1002/14651858.CD002738.pub2. PMID: 11034752 Updated.
References
References to studies included in this review
Bel 1990 {published data only}
-
- Bel EH, Timmers MC, Hermans J, Dijkman JH, Sterk PJ. The long‐term effects of nedocromil sodium and beclomethasone dipropionate on bronchial responsiveness to methacholine in nonatopic asthmatic subjects. American Review of Respiratory Disease 1990;141(1):21‐8. - PubMed
Bennati 1989 {published data only}
-
- Bennati D, Piacentini GL, Peroni DG, Sette L, Testi R, Boner AL. Changes in bronchial reactivity in asthmatic children after treatment with beclomethasone alone or in association with salbutamol. Journal of Asthma 1989;26(6):359‐64. - PubMed
Bergmann 1990 {published data only}
-
- Bergmann KC, Bauer CP, Overlack A. A placebo‐controlled blinded comparison of nedocromil sodium and beclomethasone dipropionate in bronchial asthma. Lung 1990;168:230‐9. - PubMed
-
- Bergmann KC, Bauer CP, Overlack A. A placebo‐controlled, blind comparison of nedocromil sodium and beclomethasone dipropionate in bronchial asthma. Current Medical Research & Opinion 1989;11(8):533‐42. - PubMed
Berkowitz 1998 {published data only}
-
- Berkowitz R, Rachelefsky G, Harris AG, Chen R. A comparison of triamcinolone acetonide MDI with a built‐in tube extender and beclomethasone dipropionate MDI in adult asthmatics. Chest 1998;114(3):757‐65. - PubMed
Bernstein 1999 {published data only}
-
- Bernstein D, Berkowitz R, Chervinsky P, Dvorin D, Finn A, Gross G, Karetzky M, Kemp J, Laforce C, Lumry W, Mendelson L, Nelson H, Pearlman D, Rachelefsky G, Ranter P, Repsher L, Segal A, Selner J, Settipane G, Wanderer A, Cuss F, Nolop K, Harrison J. Dose ranging study of a new steroid for asthma mometasone furate dry powder inhaler. Respiratory Medicine 1999;93:603‐12. - PubMed
Boner 1991 {published data only}
-
- Boner AL, Piacentini GL, Bonizzato C, Dattoli V, Sette L. Effect of inhaled beclomethasone dipropionate on bronchial hyperreactivity in asthmatic children during maximal allergen exposure. Pediatric Pulmonology 1991;10(1):2‐5. - PubMed
Brompton/MRC 1974 {published data only}
-
- Anonymous. Double‐blind trial comparing two dosage schedules of beclomethasone dipropionate aerosol in the treatment of chronic bronchial asthma. Preliminary report of the Brompton Hospital‐Medical Research Council Collaborative Trial. Lancet 1974;2(7876):303‐7. - PubMed
Bronsky 1998 {published data only}
-
- Bronsky E, Korenblat P, Harris AG, Chen R. Comparative clinical study of inhaled beclomethasone dipropionate and triamcinolone acetonide in persistent asthma. Annals of Allergy, Asthma, & Immunology 1998;80(4):295‐302. - PubMed
BTTA 1976 {published and unpublished data}
-
- Anonymous. A controlled trial of inhaled corticosteroids in patients receiving Prednisone tablets for asthma. British Journal of Diseases of the Chest 1976;70(2):95‐103. - PubMed
Cameron 1973 {published data only}
Carpentiere 1990 {published data only}
-
- Carpentiere G, Castello F, Marino S. Effect of beclomethasone dipropionate on the bronchial responsiveness to propranolol in asthmatics. Chest 1990;98(2):263‐5. - PubMed
Chan 1993 {published and unpublished data}
Davies 1977 {published data only}
-
- Davies G, Thomas P, Broder I, et al. Steroid‐dependent asthma treated with inhaled beclomethasone dipropionate. A long‐term study. Annals of Internal Medicine 1977;86(5):549‐53. - PubMed
De Marzo 1988 {published data only}
-
- Marzo N, Fabbri LM, Crescioli S, Plebani M, Testi R, Mapp CE. Dose‐dependent inhibitory effect of inhaled beclomethasone on late asthmatic reactions and increased responsiveness to methacholine induced by toluene diisocyanate in sensitised subjects. Pulmonary Pharmacology 1988;1(1):15‐20. - PubMed
Fahy 1998 {published data only}
-
- Fahy JV, Boushey HA. Effect of low‐dose beclomethasone dipropionate on asthma control and airway inflammation. European Respiratory Journal 1998;11(6):1240‐7. - PubMed
Fanelli 1993 {published data only}
-
- Fanelli A, Maggi E, Stendardi L, Gorini M, Duranti R, Scano G. Preventive effects of beclomethasone on histamine‐induced changes in breathing pattern in asthma. Chest 1993;103(1):122‐8. - PubMed
Fournier 1990 {published data only}
-
- Fournier M, Renon D, Roy‐Ladurie F, Pappo M, Pariente R. Bronchial tolerance to three months' inhalation of beclomethasone dipropionate. Histological and microbiological study by asthmatic patients. Presse Medicale 1990;19(31):1441‐4. - PubMed
Gaddie 1973 {published data only}
-
- Gaddie J, Reid IW, Skinner C, Petrie GR, Sinclair DJ, Palmer KN. Aerosol beclomethasone dipropionate in chronic bronchial asthma. Lancet 1973;1(7805):691‐3. - PubMed
Gross 1999 {published data only}
-
- Gross G, Thompson PJ, Chervinsky P, Vanden Burgt J. Hydrofluoroalkane‐134a beclomethasone dipropionate, 400 mug, is as effective as chlorofluorocarbon beclomethasone dipropionate, 800 mug, for the treatment of moderate asthma. Chest 1999;115(2):343‐51. - PubMed
Hampel 2000 {published data only}
-
- Hampel F, Lisberg E, Guerin JC. Effectiveness of low doses (50mcg and 100mcg bid) of beclamethasone dipropionate delivered as a CFC‐free extrafine aerosol in adults with mild‐to‐moderate asthma. Journal of Asthma 2000;37(5):389‐98. - PubMed
Harvey 1976 {published data only}
-
- Harvey LL, Nair SV, Kass I. Beclomethasone dipropionate aerosol in the treatment of steroid‐dependent asthma. A 12‐week double‐blind study comparing beclomethasone dipropionate and a vehicle aerosol. Chest 1976;70(03):345‐50. - PubMed
Hodson 1974 {published data only}
-
- Hodson ME, Batten JC, Clarke SW, Gregg I. Beclomethasone dipropionate aerosol in asthma. Transfer of steroid‐dependent asthmatic patients from oral prednisone to beclomethasone dipropionate aerosol. American Review of Respiratory Disease 1974;110(4):403‐8. - PubMed
Holst 1974 {published data only}
-
- Holst PE, O'Donnell TV. A controlled trial of beclomethasone dipropionate in asthma. New Zealand Medical Journal 1974;79(511):769‐73. - PubMed
Hoshino 1998 {published data only}
-
- Hoshino M, Nakamura Y, Sim JJ, Yamashiro Y, Uchida K, Hosaka K, Isogai S. Inhaled corticosteroid reduced lamina reticularis of the basement membrane by modulation of insulin‐like growth factor (IGF)‐I expression in bronchial asthma [see comments].. Clinical & Experimental Allergy 1998;28(5):568‐77. - PubMed
Hoshino 2001 {published data only}
-
- Hoshino M, Takahashi M, Takai Y, Sim J, Aoike N. Inhaled corticosteroids decrease vascularity of the bronchial mucosa in patients with asthma. Clinical and Experimental Allergy 2001;31:722‐30. - PubMed
Katsunuma 1993 {published data only}
-
- Katsunuma T, Hashimoto K, Akimoto K, Ebisawa M, Iikura Y. Effect of inhaled beclomethasone dipropionate on bronchial responsiveness in patients with asthma. Annals of Allergy 1993;70(2):165‐70. - PubMed
Kerigan 1977 {published data only}
Kerrebijn 1976 {published data only}
-
- Kerrebijn KF. Beclomethasone dipropionate in long‐term treatment of asthma in children. Journal of Pediatrics 1976;89(5):821‐6. - PubMed
Kerstjens 1994 {published and unpublished data}
-
- Kerstjens HA, Brand PL, Hughes MD, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. Dutch Chronic Non‐Specific Lung Disease Study Group [see comments]. New England Journal of Medicine 1992;327(20):1413‐9. - PubMed
Klein 1977 {published data only}
-
- Klein R, Waldman D, Kershnar H, et al. Treatment of chronic childhood asthma with beclomethasone dipropionate aerosol: I. A double‐blind crossover trial in nonsteroid‐dependent patients. Pediatrics 1977;60(1):7‐13. - PubMed
Kraemer 1987 {published data only}
-
- Kraemer R, Sennhauser F, Reinhardt M. Effects of regular inhalation of beclomethasone dipropionate and sodium cromoglycate on bronchial hyperreactivity in asthmatic children. Acta Paediatrica Scandinavica 1987;76(1):119‐23. - PubMed
Lacronique 1991 {published data only}
-
- Lacronique J, Renon D, Georges D, Henry‐Amar M, Marsac J. High‐dose beclomethasone: oral steroid‐sparing effect in severe asthmatic patients. European Respiratory Journal 1991;4(7):807‐12. - PubMed
Laviolette 1994 {published and unpublished data}
-
- Laviolette M, Ferland C, Trepanier L, Rocheleau H, Dakhama A, Boulet LP. Effects of inhaled steroids on blood eosinophils in moderate asthma. Annals of the New York Academy of Science 1994;725:288‐97. - PubMed
Laviolette 1999 {published data only}
-
- Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet JC, Peszek I, Zhang J, Reiss TF. Montelukast added to inhaled beclomethasone in treatment of asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(6):1862‐8. - PubMed
Lovera 1975 {published data only}
-
- Lovera J, Collins‐Williams C, Bailey J. Beclomethasone dipropionate by aerosol in the treatment in asthmatic children. Postgraduate Medical Journal 1975;51(Suppl 4):96‐8. - PubMed
-
- Lovera J, Cooper DM, Collins‐Williams C, Levison H, Bailey JD, Orange RP. Clinical and physiological assessment of asthmatic children treated with beclomethasone dipropionate. Journal of Allergy & Clinical Immunology 1976;57(2):112‐23. - PubMed
Maestrelli 1993 {published data only}
-
- Maestrelli P, Marzo N, Saetta M, Boscaro M, Fabbri LM, Mapp CE. Effects of inhaled beclomethasone on airway responsiveness in occupational asthma. Placebo‐controlled study of subjects sensitized to toluene diisocyanate. American Review of Respiratory Disease 1993;148(2):407‐12. - PubMed
Malmstrom 1999 {published data only}
-
- Malmstrom K, Rodriguez‐Gomez G, Guerra J, Villaran C, Pineiro A, Wei L, Seidenberg B, Reiss. Oral montelukast inhaled beclomethasone and placebo for chronic asthma. Annals of Internal Medicine 1999;130(6):487‐95. - PubMed
Martin 1974 {published data only}
-
- Martin PD, Gebbie T, Salmond CE. A controlled trial of beclomethasone dipropionate by aerosol in chronic asthmatics. New Zealand Medical Journal 1974;79(511):773‐6. - PubMed
Matthys 1998 {published data only}
-
- Matthys H, Nowak D, Hader S, Kunkel G. Efficacy of chlorofluorocarbon‐free beclomethasone dipropionate 400 micrograms day‐1 delivered as an extrafine aerosol in adults with moderate asthma. Respiratory Medicine 1998;92 Suppl A:17‐22. - PubMed
Messerli 1975 {published data only}
-
- Messerli C, Studer H, Scherrer M. Systemic side effects of beclomethasone dipropionate aerosols (becotide, aldecine, sanasthmyl) in otherwise non steroid treated asthmatic patients. Pneumonologie 1975;153(1):29‐42. - PubMed
Nathan 1997 {published data only}
-
- Nathan RA, Nolop KB, Cuss FM, Lorber RR. A comparison of double‐strength beclomethasone dipropionate (84 microg) MDI with beclomethasone dipropionate (42 microg) MDI in the treatment of asthma [see comments]. Chest 1997;112(1):34‐9. - PubMed
Nathan 2001 {published data only}
-
- Nathan RA, Pinnas JL, Schwartz HJ, Grossman J, Yancey SW, Emmett AH, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy 1999;82(6):521‐9. - PubMed
Nayak 2002 {published data only}
-
- Nayak A, Robert L, Weinstein S, Stampone P, Welch M. Efficacy and safety of beclomethasone dipropionate extrafine aerosol in childhood asthma a 12 ‐week randomized double blind placebo controlled study. Chest 2002;122:1956‐65. - PubMed
Pennings 1997 {published data only}
-
- Pennings HJ, Wouters EF. Effect of inhaled beclomethasone dipropionate on isocapnic hyperventilation with cold air in asthmatics, measured with forced oscillation technique. European Respiratory Journal 1997;10(3):665‐71. - PubMed
Radha 1975 {published data only}
-
- Radha TG, Viswanathan R, Sharma AK. Beclomethasone dipropionate aerosol in asthma: a double blind study. Indian Journal of Medical Research 1975;63(11):1659‐66. - PubMed
Riordan 1974 {published data only}
-
- Riordan JF, Dash CH, Sillett RW, McNicol MW. A comparison of betamethasone valerate, beclomethasone dipropionate and placebo by inhalation for the treatment of chronic asthma. Postgraduate Medical Journal 1974;50(suppl 4):61‐4. - PubMed
Ryan 1985 {published data only}
-
- Ryan G, Latimer KM, Juniper EF, Roberts RS, Hargreave FE. Effect of beclomethasone dipropionate on bronchial responsiveness to histamine in controlled nonsteroid‐dependent asthma. Journal of Allergy & Clinical Immunology 1985;75(1 Pt 1):25‐30. - PubMed
Salmeron 1989 {published data only}
-
- Salmeron S, Guerin JC, Godard P, et al. High doses of inhaled corticosteroids in unstable chronic asthma. A multicenter, double‐blind, placebo‐controlled study. American Review of Respiratory Disease 1989;140(1):167‐71. - PubMed
Simons 1997 {published data only}
-
- Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. New England Journal of Medicine 1997;337:1659‐65. - PubMed
Smith 1973a {published data only}
-
- Smith AP, Booth M, Davey AJ. A controlled trial of beclomethasone dipropionate for asthma. British Journal of Diseases of the Chest 1973;67(3):208‐14. - PubMed
Smith 1973b {published data only}
-
- Smith JM. A clinical trial of beclomethasone dipropionate aerosol in children and adolescents with asthma. Clinical Allergy 1973;3(3):249‐53. - PubMed
Toogood 1977 {published data only}
-
- Toogood JH, Lefcoe NM, Haines DS, et al. A graded dose assessment of the efficacy of beclomethasone dipropionate aerosol for severe chronic asthma. Journal of Allergy & Clinical Immunology 1977;59(4):298‐308. - PubMed
Trigg 1994 {published data only}
-
- Trigg CJ, Manolitsas ND, Wang J, et al. Placebo‐controlled immunopathologic study of four months of inhaled corticosteroids in asthma. American Journal of Respiratory & Critical Care Medicine 1994;150(1):17‐22. - PubMed
Turner 1998 {published data only}
-
- Turner MO, Johnston PR, Pizzichini E, Pizzichini MM, Hussack PA, Hargreave FE. Anti‐inflammatory effects of salmeterol compared with beclomethasone in eosinophilic mild exacerbations of asthma: A randomized, placebo controlled trial. Canadian Respiratory Journal 1998;5(4):261‐8. - PubMed
Vatrella 1996 {published data only}
-
- Ponticiello A, Vatrella A, Parrella R, et al. Inhaled beclomethasone dipropionate (BDP) prevents seasonal changes in atopic asthmatics. Monaldi Archives for Chest Disease 1997;52(2):112‐7. - PubMed
-
- Vatrella A, Ponticiello A, Parrella R, et al. Serum eosinophil cationic protein (ECP) as a marker of disease activity and treatment efficacy in seasonal asthma. Allergy 1996;51(8):547‐55. - PubMed
Vilsvik 1974 {published data only}
-
- Vilsvik JS, Schaanning J. Beclomethasone dipropionate aerosol in adult steroid‐dependent obstructive lung disease. Scandinavian Journal of Respiratory Diseases 1974;55(3):169‐75. - PubMed
Vogt 1976 {published data only}
-
- Vogt F, Chervinsky P, Dwek J, Grieco M. Beclomethasone dipropionate aerosol in the treatment of chronic bronchial asthma. Journal of Allergy & Clinical Immunology 1976;58(2):316‐21. - PubMed
Wang 1994 {published data only}
-
- Wang JH, Trigg CJ, Devalia JL, Jordan S, Davies RJ. Effect of inhaled beclomethasone dipropionate on expression of proinflammatory cytokines and activated eosinophils in the bronchial epithelium of patients with mild asthma. Journal of Allergy & Clinical Immunology 1994;94(6 Pt 1):1025‐34. - PubMed
Webb 1977 {published data only}
-
- Webb DR. Beclomethasone in steroid‐dependent asthma. Effective therapy and recovery of hypothalamo‐pituitary‐adrenal function. The Journal of the American Medical Association 1977;238(14):1508‐11. - PubMed
Wiebicke 1990 {published data only}
-
- Wiebicke W, Jorres R, Magnussen H. Comparison of the effects of inhaled corticosteroids on the airway response to histamine, methacholine, hyperventilation, and sulfur dioxide in subjects with asthma. Journal of Allergy & Clinical Immunology 1990;86(6 Pt 1):915‐23. - PubMed
References to studies excluded from this review
Apold 1975 {published data only}
-
- Apold J. [Treatment of asthma in children with beclomethasone dipropionate aerosols]. Tidsskrift for Den Norske Laegeforening 1975;95(19‐21):1149‐52. - PubMed
Baba 2002 {published data only}
-
- Baba K, Sakakibara A, Tagi T, Niwa S, Wakayama H, Takagi K.
-
- Baba K, Sakakibara A, Tagi T, Niwa S, Wakayama H, Takagi K. Long term observation of the clinical course after step down of corticosteroid inhalation in adult chronic asthmatics correlation with serum levels of eosinophil cationic protein. Respirology 2002;7:225‐66. - PubMed
Bentley 1996 {published data only}
-
- Bentley AM, Walker S, Hanotte F, Vos C, Durham SR. A comparison of the effects of oral cetirizine and inhaled beclomethasone on early and late asthmatic responses to allergen and the associated increase in airways hyperresponsiveness. Clinical & Experimental Allergy 1996;26(8):909‐17. - PubMed
Bonnaud 1991 {published data only}
-
- Bonnaud F, Desfougeres JL. [Treatment of asthma in the adult using a combination of salbutamol 100 micrograms‐‐beclomethasone 50 micrograms. Results of a study on 1917 patients seen in general medicine]. Allergie et Immunologie 1991;23(7):295‐300. - PubMed
Burge 1982 {published data only}
-
- Burge PS. The effects of corticosteroids on the immediate asthmatic reaction. European Journal of Respiratory Diseases ‐ Supplement 1982;122:163‐6. - PubMed
Chang 1998 {published data only}
Choovoravech 1977 {published data only}
-
- Choovoravech P, Choovoravech N, Yongpanich Y, Preeyasombut C. [Beclomethasone dipropionate aerosol in the treatment of chronic bronchial asthma: A preliminary study in Thai subjects]. Journal of the Medical Association of Thailand 1977;60(12):619‐25. - PubMed
Cockcroft {published data only}
-
- Cockcroft DW, McParland CP, O'Byrne PM, et al. Beclomethasone given after the early asthmatic response inhibits the late response and the increased methacholine responsiveness and cromolyn does not. Journal of Allergy and Clinical Immunology 1993;91(6):1163‐8. - PubMed
Del Bufalo 1989 {published data only}
-
- Bufalo C, Impullitti S, Miniero R, Gunella G. [Clinical controlled study on high doses of beclomethasone dipropionate associated with salbutamol in long term treatment of asthmatic subjects]. Rivista di Patologia e Clinica della Tubercolosi e di Pneumologia 1989;60:137‐55.
Douay 1976 {published data only}
-
- Douay B, Robin H, Racadot A. Study of a novel corticoid (Becotide) in chronic asthma and COPD [Étude d'un nouveau corticoïde local, la Bécotide, dans l'asthme chronique et la bronchite chronique spastique]. Lille Medical 1976;21(7 Suppl 3):623‐8. - PubMed
Doull 1995a {published data only}
-
- Doull IJ, Freezer NJ, Holgate ST. Growth of prepubertal children with mild asthma treated with inhaled beclomethasone dipropionate. American Journal of Respiratory & Critical Care Medicine 1995;151(6):1715‐9. - PubMed
-
- Doull IJM, Sandall D, Smith S, Schreiber J, Freezer NJ, Holgate ST. Differential inhibitory effect of regular inhaled corticosteroid on airway responsiveness to adenosine 5' monophosphate, methacholine, and bradykinin in symptomatic children with recurrent wheeze. Pediatric Pulmonology 1997;23(6):404‐11. - PubMed
Emel'ionov 1995 {published data only}
-
- Emel'ianov AV, Trofimov VI. [The effect of glucocorticoids on mineral metabolism in bronchial asthma patients]. Ter Arkh 1995;67(12):31‐3. - PubMed
Francia 1994 {published data only}
-
- Francia G, Senna GE, Betteli C, Musumeci C, Fratta‐Pasini A, Piubello G, et al. Pituitary‐adrenal function in asthmatic patients treated with high dose of beclomethasone. Allerg Immunol (Paris) 1994;26(1):11‐5. - PubMed
Harrison 1999 {published data only}
-
- Harrison LI, Colice GL, Donnell D, Soria I, Dockhorn R. Adrenal effects and pharmacokinetics of CFC‐free beclomethasone dipropionate: A 14‐day dose‐response study. Journal of Pharmacy & Pharmacology 1999;51(3):263‐9. - PubMed
Harvald 1976 {published data only}
-
- Harvald B, Letman H. [Treatment of asthma with beclomethasone]. Ugeskrift for Laeger 1976;138(44):2717‐21. - PubMed
Hoshino 1999 {published data only}
-
- Hoshino M, Takahashi M, Takai Y, JaeJoon S. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 expression in asthma. Journal of Allergy and Clinical Immunology 1999;104(2):356‐63. - PubMed
Huang 1993 {published data only}
-
- Huang JL, Hung IJ, Hsieh KH. Effect of inhaled beclomethasone dipropionate in the treatment of recurrent wheezing in infancy and early childhood. Journal of the Formosan Medical Association 1993;92(12):1066‐9. - PubMed
Kaptein 1993 {published data only}
-
- Kaptein AA, Brand PL, Dekker FW, Kerstjens HA, Postma DS, Sluiter HJ. Quality‐of‐life in a long‐term multicentre trial in chronic nonspecific lung disease: assessment at baseline. The Dutch CNSLD Study Group. European Respiratory Journal 1993;6:1479‐84. - PubMed
Konig 1974 {published data only}
-
- Konig P, Jaffe P, Godfrey S. Effect of corticosteroids on exercise‐induced asthma. Journal of Allergy & Clinical Immunology 1974;54(1):14‐9. - PubMed
Malo 1996 {published and unpublished data}
-
- Malo JL, Cartier A, Cote J, et al. Influence of inhaled steroids on recovery from occupational asthma after cessation of exposure: an 18‐month double‐blind crossover study. American Journal of Respiratory & Critical Care Medicine 1996;153(3):953‐60. - PubMed
Menz 1986 {published data only}
-
- Menz G. [Treatment of asthma with high doses of beclomethasone]. Schweizerische Rundschau fur Medizin Praxis 1986;75(30‐31):914‐6. - PubMed
Michel 1977 {published data only}
-
- Michel FB, Clauzel AM, Calvayrac P, Grenier J. [Mechanisms of action of glucocorticoids in the treatment of asthma. Comparative effects of glucocorticoids via the general route and beclomethasone dipropionate via aerial route on plasma levels of cyclic AMP]. Nouvelle Presse Medicale 1977;6(15):1278‐80. - PubMed
Nakajima 1979 {published data only}
-
- Nakajima S, Fujihara Y, Tsuya Y, Ohishi M, Takagi O. Changes of bronchoconstriction by treatment. Acta Medica Kinki University 1979;4(2):273‐9.
Nielsen 1999 {published data only}
-
- Nielsen LP, Pedersen B, Faurschou P, Madsen F, Wilcke JTR, Dahl R. Salmeterol reduces the need for inhaled corticosteroid in steroid‐dependent asthmatics. Respiratory Medicine 1999;93(12):863‐8. - PubMed
Nizankowska 1989 {published data only}
-
- Nizankowska E, Szczeklik A. Glucocorticosteroids attenuate aspirin‐precipitated adverse reactions in aspirin‐intolerant patients with asthma. Annals of Allergy 1989;63:159‐62. - PubMed
Noonan 2001 {published data only}
-
- Noonan I, Reiss T, Knorr B, Guerra J, Matz J. Long term asthma control with oral montelukast and inhaled beclomethasone for adults and children 6 yeras and older. Clinical & Experimental Allergy 2001;31:845‐45. - PubMed
Patakas 1978 {published data only}
-
- Patakas DLGSL. Effect of beclomethasone dipropionate inhalation on exercise induced bronchospasm. IRCS Medical Science: Cardiovascular System 1978;6(11):448.
Pizzichini 1996 {published and unpublished data}
-
- Pizzichini MM, Kidney JC, Wong BJ, et al. Effect of salmeterol compared with beclomethasone on allergen‐induced asthmatic and inflammatory responses [published erratum appears in Eur Respir J 1996 Oct;9(10):2190]. European Respiratory Journal 1996;9(3):449‐55. - PubMed
Richards 1978 {published data only}
-
- Richards W, Platzker A, Church JA, Yamamoto F, Foster S. Steroid‐dependent asthma treated with inhaled beclomethasone dipropionate in children. Annals of Allergy 1978;41(5):274‐7. - PubMed
Rodriguez 1986 {published data only}
-
- Rodriguez Sanchon B, Manresa F, Romero P, Cardona M. [Nocturnal asthma: Treatment with high inhalatory doses of salbutamol and beclomethasone]. Revista Clinica Espanola 1986;178:322‐6. - PubMed
Rohdewald 1992 {published data only}
-
- Rohdewald P. [Inhaled glucocorticoids. Slight side effects at high effectiveness]. Deutsche Medizinische Wochenschrift 1992;117(10):390‐3. - PubMed
Ronchetti 1976 {published data only}
-
- Ronchetti R, Gentili G. [Beclomethasone in the treatment of infantile asthma]. Minerva Pediatrica 1976;28(7):478‐85. - PubMed
Ruff 1977 {published data only}
-
- Ruff F, Brouet G. [Four years use of beclomethasone dipropionate aerosols]. Nouvelle Presse Medicale 1977;6(15):1283‐5. - PubMed
Sidel'nikov 1985 {published data only}
-
- Sidel'nikov VM, Pashun TV, Pomytkina LR. [Comparative evaluation of the effectiveness of different methods of prophylactic treatment for children with bronchial asthma]. Pediatriia 1985;4:34‐6. - PubMed
Sil'vestrov 1983 {published data only}
-
- Sil'vestrov VP, Surovov Iu A, Pakulin IA, Larin NV. [Effect of treatment with ventolin and becotide on ventilation and hemodynamics in patients with bronchial asthma]. Klinicheskaia Meditsina 1983;61(12):54‐8. - PubMed
Silkoff 2001 {published data only}
-
- Silkoff P, McClean P, Spino M, Erlich L, Slutsky A, Zamel N. Dose response relationship and peroducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest 2001;119:1322‐28. - PubMed
Stick 1995 {published data only}
Storr 1986 {published data only}
Webb 1986 {published data only}
References to studies awaiting assessment
Boszormeny‐Nagy 1980 {published data only}
-
- Boszormenyi‐Nagy G, Herjavecz I. [Clinical effects of beclomethasone dipropionate in bronchial asthma]. Orvosi Hetilap 1980;121(16):947‐50. - PubMed
Chonabayashi 1986 {published data only}
-
- Chonabayashi N, Yoshimura K, Nakamori Y, Nakatani T, Nakata K, Tanimoto H. [Method of decreasing oral corticosteroids by adding a beclomethasone dipropionate inhaler for steroid‐dependent asthmatic patients]. Nippon Kyobu Shikkan Gakkai Zasshi ‐ Japanese Journal of Thoracic Diseases 1986;24(5):522‐30. - PubMed
Iwata 1995 {published data only}
-
- Iwata M, Iinuma Y, Sugino Y, Suzuki Y. [Effects and limitations of inhaled high dose beclomethasone dipropionate (2400 micrograms/day) in steroid‐dependent asthmatics]. Arerugi 1995;44(5):562‐6. - PubMed
Kudo 1995 {published data only}
-
- Kudo K, Hojo M, Kabe J. [Inhaled beclomethasone in long‐term management of asthma: optimal dose and optimal duration of treatment]. Nippon Kyobu Shikkan Gakkai Zasshi 1995;33(9):956‐65. - PubMed
Muittari 1974 {published data only}
-
- Muittari A, Veneskoski T. [Beclomethasone aerosol in asthma therapy]. Duodecim 1974;90(18):1228‐34. - PubMed
Rozniecki 1988 {published data only}
-
- Rozniecki J, Szmidt M, Grzelewska‐Rzymowska I, Gorski P, Grabski W. [Clinical evaluation of beclomethasone dipropionate aerosol in patients with bronchial asthma]. Polski Tygodnik Lekarski 1988;43(12‐13):412‐5. - PubMed
Shen 1991 {published data only}
-
- Shen X, Niu SF, Cai YY. [A clinical trial of treating asthma of moderate severity with beclomethasone dipropionate aerosol]. Chung‐Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 1991;30(9):536‐8, 593. - PubMed
Additional references
BTS 1997
-
- British Thoracic Society. The British Guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐S21.
BTS 2003
Busse 1999
-
- Busse W, brazinsky Sh, Jacobson K, stricker W, et al. Efficacy response of inhaled beclomethasone dipropionate in asthma is proportional to dose and is improved by formulation with a new propellant. Journal of Allergy and Clinical Immunology 1999;104(6):1215‐22. - PubMed
CONSORT 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. The Journal of the American Medical Association 1996;276:637‐9. - PubMed
GINA 1995
-
- National Asthma Education and Prevention Program. Global strategy for asthma management and prevention NHBLI/WHO workshop report. National Institute of Health, Bethseda, MD 1995, issue NIH Publication No. 95‐3659.
Higgins 2003
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Lipworth 1993
-
- Lipworth BJ. Clinical pharmacology of corticosteroids in bronchial asthma. Pharmacology Therapy 1993;58:173. - PubMed
Malouf 2004
-
- Malouf R, Wright J. Pressurised‐metred does inhalers versus hand‐held inhalers for the delivery of corticosteroids in asthma. The Cochrane Library 2004, Issue 4.
NHLBI 1997
-
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue Publication No. 97‐4051.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

