Weight related differences in the pharmacokinetics of abacavir in HIV-infected patients
- PMID: 15676040
- PMCID: PMC1884744
- DOI: 10.1111/j.1365-2125.2004.02259.x
Weight related differences in the pharmacokinetics of abacavir in HIV-infected patients
Abstract
Aim: To study the possible influence of patient characteristics on abacavir pharmacokinetics.
Methods: A population pharmacokinetic model for abacavir was developed using data from 188 adult patients by the use of a nonlinear mixed effects modelling method performed with NONMEM.
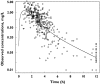
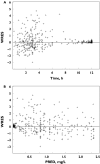
Results: Abacavir pharmacokinetics was well described by a two-compartment open model with linear absorption and elimination. Typical population estimates for the absorption rate constant (Ka), the apparent central distribution volume (Vc/F), the apparent peripheral distribution volume (Vp/F), the apparent intercompartmental clearance (Q/F) and the apparent plasma clearance (CL/F) were 1.8 h(-1), 75 l, 23.6 l, 10 l h(-1) and 47.5 l h(-1), respectively. Apparent plasma clearance was positively related to bodyweight. Individual Bayesian estimates of CL/F were used to calculate abacavir AUC. The latter decreased from 10.7 +/- 5.0 to 5.7 +/- 1.6 mgh l(-1) when bodyweight increased from 36 to 102 kg. This drop in abacavir exposure could lead to suboptimal treatment for the heaviest patients, as antiviral efficacy of abacavir is known to be related to its AUC. A 400 mg abacavir dose would be necessary to achieve adequate exposure to abacavir in patients weighing more than 60 kg.
Conclusions: The apparent plasma clearance of abacavir was positively related to bodyweight. The efficacy of the current recommended abacavir dosage for patients with high bodyweight should be evaluated in further studies.
Figures

Similar articles
-
A review of the pharmacokinetics of abacavir.Clin Pharmacokinet. 2008;47(6):351-71. doi: 10.2165/00003088-200847060-00001. Clin Pharmacokinet. 2008. PMID: 18479171 Review.
-
Abacavir pharmacokinetics in human immunodeficiency virus-infected children ranging in age from 1 month to 16 years: a population analysis.J Clin Pharmacol. 2005 Mar;45(3):257-64. doi: 10.1177/0091270004272215. J Clin Pharmacol. 2005. PMID: 15703361
-
Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy.Antimicrob Agents Chemother. 2005 Aug;49(8):3361-6. doi: 10.1128/AAC.49.8.3361-3366.2005. Antimicrob Agents Chemother. 2005. PMID: 16048948 Free PMC article.
-
Population pharmacokinetics and maximum a posteriori probability Bayesian estimator of abacavir: application of individualized therapy in HIV-infected infants and toddlers.Br J Clin Pharmacol. 2012 Apr;73(4):641-50. doi: 10.1111/j.1365-2125.2011.04121.x. Br J Clin Pharmacol. 2012. PMID: 21988586 Free PMC article.
-
Once-daily abacavir in place of twice-daily administration.Ann Pharmacother. 2005 Jul-Aug;39(7-8):1302-8. doi: 10.1345/aph.1E680. Epub 2005 Jun 14. Ann Pharmacother. 2005. PMID: 15956231 Review.
Cited by
-
Age-related differences in the pharmacokinetics of stavudine in 272 children from birth to 16 years: a population analysis.Br J Clin Pharmacol. 2007 Jul;64(1):105-9. doi: 10.1111/j.1365-2125.2007.02854.x. Epub 2007 Feb 26. Br J Clin Pharmacol. 2007. PMID: 17324223 Free PMC article.
-
A review of the pharmacokinetics of abacavir.Clin Pharmacokinet. 2008;47(6):351-71. doi: 10.2165/00003088-200847060-00001. Clin Pharmacokinet. 2008. PMID: 18479171 Review.
-
Pharmacology of current and promising nucleosides for the treatment of human immunodeficiency viruses.Antiviral Res. 2006 Sep;71(2-3):322-34. doi: 10.1016/j.antiviral.2006.03.012. Epub 2006 Apr 18. Antiviral Res. 2006. PMID: 16716415 Free PMC article. Review.
-
Population pharmacokinetics of abacavir in pregnant women.Antimicrob Agents Chemother. 2014 Oct;58(10):6287-9. doi: 10.1128/AAC.03469-14. Epub 2014 Jul 28. Antimicrob Agents Chemother. 2014. PMID: 25070097 Free PMC article.
-
Steady-state pharmacokinetics of abacavir in plasma and intracellular carbovir triphosphate following administration of abacavir at 600 milligrams once daily and 300 milligrams twice daily in human immunodeficiency virus-infected subjects.Antimicrob Agents Chemother. 2009 Apr;53(4):1532-8. doi: 10.1128/AAC.01000-08. Epub 2009 Feb 2. Antimicrob Agents Chemother. 2009. PMID: 19188387 Free PMC article. Clinical Trial.
References
-
- Harris M, Back D, Kewn S, Jutha S, Marina R, Montaner JS. Intracellular carbovir triphosphate levels in patients taking abacavir once a day. AIDS. 2002;16(8):1196–7. - PubMed
-
- Saag MS, Sonnerborg A, Torres RA, et al. Antiretroviral effect and safety of abacavir alone and in combination with zidovudine in HIV-infected adults. Abacavir Phase 2 Clin Team AIDS. 1998;12(16):F203–9. - PubMed
-
- Staszewski S, Katlama C, Harrer T, et al. A dose-ranging study to evaluate the safety and efficacy of abacavir alone or in combination with zidovudine and lamivudine in antiretroviral treatment-naive subjects. AIDS. 1998;12(16):F197–202. - PubMed
-
- Zhou XJ, Sheiner LB, D'Aquila RT, et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators Antimicrob Agents Chemother. 1999;43(1):121–8. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

