Monocyte-mediated T-cell suppression and augmented monocyte tryptophan catabolism after human hematopoietic stem-cell transplantation
- PMID: 15677560
- PMCID: PMC1895091
- DOI: 10.1182/blood-2004-05-1726
Monocyte-mediated T-cell suppression and augmented monocyte tryptophan catabolism after human hematopoietic stem-cell transplantation
Abstract
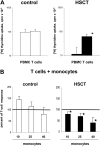
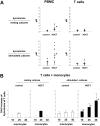
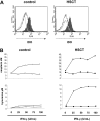
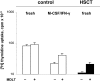
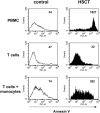
T-cell dysfunction after human hematopoietic stem-cell transplantation (HSCT) is generally attributed to intrinsic T-cell defects. Here we show that the characteristic impaired proliferative responses to polyclonal stimulation of post-HSCT peripheral blood mononuclear cells (PB-MCs) were markedly (4-fold) improved by T-cell enrichment. Conversely, addback of post-HSCT monocytes to these enriched T cells dampened their proliferative responses, suggesting that post-HSCT monocytes effectively mediate T-cell suppression. As a mechanism possibly contributing to monocyte-mediated T-cell suppression, we investigated monocyte tryptophan catabolism by indoleamine 2,3-dioxygenase into kynurenine, which has been implicated in regulating T-cell responses. Compared with controls, all post-HSCT monocyte-containing cell cultures (total PBMCs, monocytes, and monocyte/T-cell cocultures), but not monocyte-depleted populations, secreted elevated amounts of kynurenine. Blockade of tryptophan catabolism improved the proliferative responses. The slightly increased kynurenine release and substantial release of neopterin by unstimulated post-HSCT monocytes suggests that they were in a state of continuous activation. Superimposed on this state, stimulation of these cells caused a striking, additional increase (10-fold) in kynurenine release, and they triggered marked apoptosis of autologous post-HSCT T cells. We conclude that the amplified kynurenine release by post-HSCT monocytes, particularly induced upon stimulation, may underlie their suppressor activity, which in turn may contribute to the depressed T-cell immune responses after HSCT.
Figures

Similar articles
-
Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites.J Exp Med. 2002 Aug 19;196(4):447-57. doi: 10.1084/jem.20020052. J Exp Med. 2002. PMID: 12186837 Free PMC article.
-
FcepsilonRI induces the tryptophan degradation pathway involved in regulating T cell responses.J Immunol. 2002 Aug 15;169(4):1810-6. doi: 10.4049/jimmunol.169.4.1810. J Immunol. 2002. PMID: 12165503
-
Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells.Blood. 2005 Feb 15;105(4):1574-81. doi: 10.1182/blood-2004-06-2089. Epub 2004 Oct 5. Blood. 2005. PMID: 15466932
-
Tumor immune escape mediated by indoleamine 2,3-dioxygenase.Immunol Lett. 2007 Aug 15;111(2):69-75. doi: 10.1016/j.imlet.2007.06.001. Epub 2007 Jul 2. Immunol Lett. 2007. PMID: 17644189 Review.
-
Indoleamine 2,3-dioxygenase in hematopoietic stem cell transplantation.Curr Drug Metab. 2007 Apr;8(3):267-72. doi: 10.2174/138920007780362554. Curr Drug Metab. 2007. PMID: 17430114 Review.
Cited by
-
Reconstitution of the immune system and clinical correlates after stem cell transplantation for systemic sclerosis.Front Immunol. 2022 Aug 11;13:941011. doi: 10.3389/fimmu.2022.941011. eCollection 2022. Front Immunol. 2022. PMID: 36032076 Free PMC article. Review.
-
The kynurenine pathway in stem cell biology.Int J Tryptophan Res. 2013 Sep 15;6:57-66. doi: 10.4137/IJTR.S12626. Int J Tryptophan Res. 2013. PMID: 24092986 Free PMC article. Review.
-
Indoleamine 2,3-dioxygenase in human hematopoietic stem cell transplantation.Int J Tryptophan Res. 2010;3:77-90. doi: 10.4137/ijtr.s4076. Epub 2010 Jun 10. Int J Tryptophan Res. 2010. PMID: 22084590 Free PMC article.
-
Granulocyte colony-stimulating factor-induced immature myeloid cells inhibit acute graft-versus-host disease lethality through an indoleamine dioxygenase-independent mechanism.Immunology. 2009 Sep;128(1 Suppl):e632-40. doi: 10.1111/j.1365-2567.2009.03048.x. Epub 2009 Jan 12. Immunology. 2009. PMID: 19740324 Free PMC article.
-
Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy.J Hematol Oncol. 2023 Jun 5;16(1):59. doi: 10.1186/s13045-023-01453-1. J Hematol Oncol. 2023. PMID: 37277776 Free PMC article. Review.
References
-
- Fehse N, Fehse B, Kroger N, et al. Influence of anti-thymocyte globulin as part of the conditioning regimen on immune reconstitution following matched related bone marrow transplantation. J Hematother Stem Cell Res. 2003;12: 237-242. - PubMed
-
- Maris M, Boeckh M, Storer B, et al. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol. 2003;31: 941-952. - PubMed
-
- Daly A, McAfee S, Dey B, et al. Nonmyeloablative bone marrow transplantation: infectious complications in 65 recipients of HLA-identical and mismatched transplants. Biol Blood Marrow Transplant. 2003;9: 373-382. - PubMed
-
- Gur H, Krauthgamer R, Berrebi A, et al. Tolerance induction by megadose hematopoietic progenitor cells: expansion of veto cells by short-term culture of purified human CD34(+) cells. Blood. 2002;99: 4174-4181. - PubMed
-
- George JF, Lu A, Thomas JM, Kirklin JK, Pinderski LJ. Allogeneic bone marrow inhibits T-cell activation and clonal expansion in vitro. Transplantation. 2003;76: 237-243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

