SCL/Tal-1 is essential for hematopoietic commitment of the hemangioblast but not for its development
- PMID: 15677567
- PMCID: PMC1895073
- DOI: 10.1182/blood-2004-09-3611
SCL/Tal-1 is essential for hematopoietic commitment of the hemangioblast but not for its development
Abstract
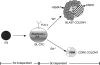
In this report, we have defined the stage at which Scl functions in the establishment of the hematopoietic system and provide evidence that its primary role is in the generation of the hematopoietic lineages from a progenitor called the blast colony-forming cell (BL-CFC), a cell considered to be the in vitro equivalent of the hemangioblast. Using an embryonic stem (ES) cell line in which lacZ cDNA has been targeted to the Scl locus, we show that most of the BL-CFCs are detected in the SCL/lacZ- population, indicating that this progenitor does not express Scl. In the blast colony assay, Scl-/- cells initiate colony growth but are unable to generate endothelial and hematopoietic progeny and thus form colonies consisting of vascular smooth muscle cells only. The capacity to give rise to blast colonies can be rescued by retroviral transduction of a wild-type Scl gene into Scl-/- FLK-1+ cells, suggesting that the BL-CFC is generated in this population. Finally, we show that Scl-/- endothelial cells display a growth deficiency in monolayer cultures that can be partially overcome by maintaining this population as 3-dimensional aggregates indicating that specific cellular interactions are required for maintenance of the Scl-/- endothelial lineage in vitro.
Figures

 ) were scored after 4 days in culture. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of the PS, SCL-, and SCL+ fractions. Each lane represents the amplified products from 1000 single cells deposited directly into lysis buffer. The 3′ cDNA was prepared and analyzed as described., (D) Total blast colonies in each fraction. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (E) FLK-1+ SCL/lacZ- cells were isolated from day 3 EBs and replated into hemangioblast cultures. Shown is colony morphology at day 1, the core stage; day 2, the early hematopoietic stage; and day 3, the blast colony stage. Arrow indicates the developing vascular core at the day 2 early hematopoietic stage of blast colony development. Original magnification, × 400. Objective × 40, numerical aperture 0.50. MagnaFire software (Optronics, Goleta, CA) was used to analyze the images. (F) Expression analyses of the starting population and of colonies at the 3 different developmental stages; 105 FLK-1+ SCL/lacZ- (F+ S-) cells, 1000 day 1 and day 2 pooled colonies and 500 day 3 pooled colonies were used for this analysis. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. Hybridization to the ribosomal gene L-32 was included to control for the level of cDNA in each lane.
) were scored after 4 days in culture. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of the PS, SCL-, and SCL+ fractions. Each lane represents the amplified products from 1000 single cells deposited directly into lysis buffer. The 3′ cDNA was prepared and analyzed as described., (D) Total blast colonies in each fraction. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (E) FLK-1+ SCL/lacZ- cells were isolated from day 3 EBs and replated into hemangioblast cultures. Shown is colony morphology at day 1, the core stage; day 2, the early hematopoietic stage; and day 3, the blast colony stage. Arrow indicates the developing vascular core at the day 2 early hematopoietic stage of blast colony development. Original magnification, × 400. Objective × 40, numerical aperture 0.50. MagnaFire software (Optronics, Goleta, CA) was used to analyze the images. (F) Expression analyses of the starting population and of colonies at the 3 different developmental stages; 105 FLK-1+ SCL/lacZ- (F+ S-) cells, 1000 day 1 and day 2 pooled colonies and 500 day 3 pooled colonies were used for this analysis. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. Hybridization to the ribosomal gene L-32 was included to control for the level of cDNA in each lane.
 ) were scored from the unfractionated (PS indicates presort), F-S-, F+ S-, F+ S+, and F-S+ populations. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of isolated fractions. Each lane represents the amplified products from 1000 single cells. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Total number of hematopoietic colonies in each of the indicated fractions. Colonies were categorized as follows: ERY/P (
) were scored from the unfractionated (PS indicates presort), F-S-, F+ S-, F+ S+, and F-S+ populations. Data are presented as mean number of blast colonies from 3 dishes. Bars, where visible, represent SEM. (C) Expression analyses of isolated fractions. Each lane represents the amplified products from 1000 single cells. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Total number of hematopoietic colonies in each of the indicated fractions. Colonies were categorized as follows: ERY/P ( ), primitive erythroid; MAC (□), macrophages; E/MAC (
), primitive erythroid; MAC (□), macrophages; E/MAC ( ), erythroid-macrophage; and MIX (
), erythroid-macrophage; and MIX ( ), multilineage. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM.
), multilineage. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM.
 , MIX. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression of the endogenous and viral Scl transcripts in day 3.5 Scl+/+ FLK-1+ (sort, +/+), day 4 Scl-/- FLK-1+ (sort, -/-), and 2 pools of rescued Scl-/- FLK-1+ cells (rescue, -/-) harvested following 3 days of coculture with SCL virus-producing cells (SCLVPCs) and 5 days of expansion in KL, IL-3, and EPO. To facilitate the detection of endogenous Scl, primers were designed against the 3′ UTR of the Scl gene. Detection of viral Scl was carried out using a 5′ primer designed against the 3′ translated region of the Scl gene and a 3′ primer designed against the PGK promoter present only in the retroviral transcript. (E) Expression analyses of the populations described in panel D were performed using probes designed against the 3′ UTR of the indicated genes.
, MIX. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression of the endogenous and viral Scl transcripts in day 3.5 Scl+/+ FLK-1+ (sort, +/+), day 4 Scl-/- FLK-1+ (sort, -/-), and 2 pools of rescued Scl-/- FLK-1+ cells (rescue, -/-) harvested following 3 days of coculture with SCL virus-producing cells (SCLVPCs) and 5 days of expansion in KL, IL-3, and EPO. To facilitate the detection of endogenous Scl, primers were designed against the 3′ UTR of the Scl gene. Detection of viral Scl was carried out using a 5′ primer designed against the 3′ translated region of the Scl gene and a 3′ primer designed against the PGK promoter present only in the retroviral transcript. (E) Expression analyses of the populations described in panel D were performed using probes designed against the 3′ UTR of the indicated genes.
 , 2° EBs; ▪, BLASTS). Numbers below the graph indicate the day of differentiation. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (B) Blast colony potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 3.5, 4, 5, and 6 Scl-/- EBs (-/-, 3.5/4/5/6). Colony-forming potential was measured in the various FLK-1+ populations prior to (- virus) and following infection with SCLVPCs (+ virus). Colony numbers are presented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (C) Hematopoietic progenitor potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 4, 5, and 6 Scl-/- EBs (-/-, 4/5/6). Colony-forming potential was measured prior to (- virus) and following infection with SCLVPCs (+ virus). □ indicates ERY/P; ▪, MAC;
, 2° EBs; ▪, BLASTS). Numbers below the graph indicate the day of differentiation. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (B) Blast colony potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 3.5, 4, 5, and 6 Scl-/- EBs (-/-, 3.5/4/5/6). Colony-forming potential was measured in the various FLK-1+ populations prior to (- virus) and following infection with SCLVPCs (+ virus). Colony numbers are presented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (C) Hematopoietic progenitor potential of FLK-1+ cells from day 3.5 Scl+/+ EBs (+/+, 3.5) and FLK-1+ cells from day 4, 5, and 6 Scl-/- EBs (-/-, 4/5/6). Colony-forming potential was measured prior to (- virus) and following infection with SCLVPCs (+ virus). □ indicates ERY/P; ▪, MAC;  , E/MAC; and
, E/MAC; and  , MIX. Colony numbers are represented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression analyses of the presorted (P), FLK-1- (-) and FLK-1+ (+) fractions from day 3.5 Scl+/+ EBs (+/+) and from the different stage Scl-/- EBs (-/-). Boxes highlight Brachyury expression in FLK-1+ fractions from Scl+/+ and Scl-/- EBs at the indicated time points (in days) shown below the figure.
, MIX. Colony numbers are represented per 105 input cells. Data are presented as mean number of colonies from 3 dishes. Bars, where visible, represent SEM. (D) Expression analyses of the presorted (P), FLK-1- (-) and FLK-1+ (+) fractions from day 3.5 Scl+/+ EBs (+/+) and from the different stage Scl-/- EBs (-/-). Boxes highlight Brachyury expression in FLK-1+ fractions from Scl+/+ and Scl-/- EBs at the indicated time points (in days) shown below the figure.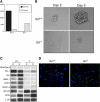
 ) development from FLK-1+ day 3 Scl+/+ and day 3.5 Scl-/- EBs. Data are presented as mean number of blast colonies and cores per 105 input cells from 3 dishes. (B) Photographs of Scl+/+ blast colonies and Scl-/- cores at day 2 and 3 of development. Original magnification, × 200. Objective × 20, numerical aperture 0.30. MagnaFire software was used to analyze the images. (C) RT-PCR analysis of Scl+/+ (+/+) and Scl-/- (-/-) colonies at days 2 and 3 of growth. Each lane represents a pool of 500 colonies. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Immunohistochemistry of adhesive cells generated from Scl+/+ blast colonies (Scl+/+) and Scl-/- cores (Scl-/-). CD31+ (red) and SMA+ (green) populations are indicated. Original magnification, × 200. Objective × 20, numerical aperture 0.70. OpenLab software was used to analyze the images.
) development from FLK-1+ day 3 Scl+/+ and day 3.5 Scl-/- EBs. Data are presented as mean number of blast colonies and cores per 105 input cells from 3 dishes. (B) Photographs of Scl+/+ blast colonies and Scl-/- cores at day 2 and 3 of development. Original magnification, × 200. Objective × 20, numerical aperture 0.30. MagnaFire software was used to analyze the images. (C) RT-PCR analysis of Scl+/+ (+/+) and Scl-/- (-/-) colonies at days 2 and 3 of growth. Each lane represents a pool of 500 colonies. Hybridization was carried out with probes designed against the 3′ UTR of the indicated genes. (D) Immunohistochemistry of adhesive cells generated from Scl+/+ blast colonies (Scl+/+) and Scl-/- cores (Scl-/-). CD31+ (red) and SMA+ (green) populations are indicated. Original magnification, × 200. Objective × 20, numerical aperture 0.70. OpenLab software was used to analyze the images.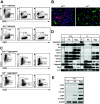
Similar articles
-
Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression.Development. 2002 Dec;129(23):5511-20. doi: 10.1242/dev.00149. Development. 2002. PMID: 12403720
-
A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells.Development. 2004 Jun;131(11):2749-62. doi: 10.1242/dev.01130. Development. 2004. PMID: 15148304
-
Basic fibroblast growth factor positively regulates hematopoietic development.Development. 2000 May;127(9):1931-41. doi: 10.1242/dev.127.9.1931. Development. 2000. PMID: 10751181
-
Regulation of hemangioblast development.Ann N Y Acad Sci. 2001 Jun;938:96-107; discussion 108. doi: 10.1111/j.1749-6632.2001.tb03578.x. Ann N Y Acad Sci. 2001. PMID: 11458531 Review.
-
Developmental relationship between hematopoietic and endothelial cells.Immunol Res. 2005;32(1-3):57-74. doi: 10.1385/IR:32:1-3:057. Immunol Res. 2005. PMID: 16106059 Review.
Cited by
-
The balance of positive and negative effects of TGF-β signaling regulates the development of hematopoietic and endothelial progenitors in human pluripotent stem cells.Stem Cells Dev. 2013 Oct 15;22(20):2765-76. doi: 10.1089/scd.2013.0008. Epub 2013 Jul 19. Stem Cells Dev. 2013. PMID: 23758278 Free PMC article.
-
Notch Signaling in HSC Emergence: When, Why and How.Cells. 2022 Jan 21;11(3):358. doi: 10.3390/cells11030358. Cells. 2022. PMID: 35159166 Free PMC article. Review.
-
Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs.Cell Stem Cell. 2008 Jul 3;3(1):55-68. doi: 10.1016/j.stem.2008.04.004. Cell Stem Cell. 2008. PMID: 18593559 Free PMC article.
-
Structural basis for LMO2-driven recruitment of the SCL:E47bHLH heterodimer to hematopoietic-specific transcriptional targets.Cell Rep. 2013 Jul 11;4(1):135-47. doi: 10.1016/j.celrep.2013.06.008. Epub 2013 Jul 3. Cell Rep. 2013. PMID: 23831025 Free PMC article.
-
MiR-24 is required for hematopoietic differentiation of mouse embryonic stem cells.PLoS Genet. 2015 Jan 29;11(1):e1004959. doi: 10.1371/journal.pgen.1004959. eCollection 2015 Jan. PLoS Genet. 2015. PMID: 25634354 Free PMC article.
References
-
- Murray PDF. The development of in vitro of the blood of the early chick embryo. Proc Roy Soc London. 1932;11: 497-521.
-
- Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80: 575-581. - PubMed
-
- Millauer B, Wizigmann-Voos S, Schnürch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72: 835-846. - PubMed
-
- Kallianpur AR, Jordan JE, Brandt SJ. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood. 1994;83: 1200-1208. - PubMed
-
- Watt SM, Gschmeissner SE, Bates PA. PECAM-1: its expression and function as a cell adhesion molecule on hemopoietic and endothelial cells. Leuk Lymphoma. 1995;17: 229-244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

