Excitatory purinergic neurotransmission in smooth muscle of guinea-pig [corrected] taenia caeci
- PMID: 15677692
- PMCID: PMC1665602
- DOI: 10.1113/jphysiol.2004.077636
Excitatory purinergic neurotransmission in smooth muscle of guinea-pig [corrected] taenia caeci
Erratum in
- J Physiol. 2005 May 1;564(Pt 3):953
Abstract
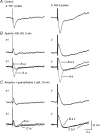
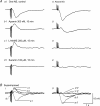
Non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmission has been an area of intense interest in gut motor physiology, whereas excitatory NANC neurotransmission has received less attention. In order to further explore excitatory NANC neurotransmission, we performed conventional intracellular recordings from guinea-pig taenia caeci smooth muscle. Tissue was perfused with oxygenated Krebs solution at 35 degrees C and nerve responses evoked by either oral or aboral nerve stimulation (NS) (4 square wave pulses, 0.3 ms duration, 20 Hz). Electrical activity was characterized by slow waves upon which one to three action potentials were superimposed. Oral NS evoked an inhibitory junction potential (IJP) at either the valley or peak of the slow wave. Application of nifedipine (1 microM) abolished slow waves and action potentials, but membrane potential flunctuations (1-3 mV) and IJPs remained unaffected. Concomitant application of apamin (300 nM), a small-conductance Ca(2+)-activated K(+) channel blocker, converted the IJP to an EJP that was followed by slow IJP. Further administration of N(G)-nitro-l-arginine methyl ester (l-NAME, 200 microM), a nitric oxide synthase inhibitor, abolished the slow IJP without affecting the EJP, implying that the slow IJP is due to nitrergic innervation. The EJP was abolished by tetrodotoxin (1 microM), but was not significantly affected by atropine (3 microM) and guanethidine (3 microM) or hexamethonium (500 microM). Substance P (SP, 1 microM) desensitization caused slight attenuation of the EJP, but the EJP was abolished by desensitization with alpha,beta-methylene ATP (50 microM), a P2 purinoceptor agonist that is more potent than ATP at the P2X receptor subtype, suramin (100 microM), a non-selective P2 purinoceptor antagonist, and pyridoxal-phosphate-6-azophenyl-2',4'-disulphonic acid (PPADS, 100 microM) , a selective P2X purinoceptor antagonist. In contrast, the EJP was unaffected by MRS-2179 (2 microM), a selective P2Y(1) receptor antagonist. Aboral NS evoked an apamin- and l-NAME-sensitive IJP, but virtually no NANC EJP. These data suggest the presence of polarized excitatory purinergic neurotransmission in guinea-pig taenia caeci, which appears to be mediated by P2X purinoceptors, most likely the P2X(1) subtype.
Figures







Similar articles
-
NANC inhibitory neuromuscular transmission in the hamster distal colon.Pharmacol Res. 2006 Dec;54(6):452-60. doi: 10.1016/j.phrs.2006.09.004. Epub 2006 Sep 17. Pharmacol Res. 2006. PMID: 17035041
-
The possible role of ATP and PACAP as mediators of apaminsensitive NANC inhibitory junction potentials in circular muscle of guinea-pig colon.Br J Pharmacol. 1996 Nov;119(5):779-86. doi: 10.1111/j.1476-5381.1996.tb15740.x. Br J Pharmacol. 1996. PMID: 8922721 Free PMC article.
-
P2Y1 receptors mediate apamin-sensitive and -insensitive inhibitory junction potentials in murine colonic circular smooth muscle.J Pharmacol Exp Ther. 2010 May;333(2):602-11. doi: 10.1124/jpet.109.160978. Epub 2010 Jan 26. J Pharmacol Exp Ther. 2010. PMID: 20103587
-
Design and pharmacology of selective P2-purinoceptor antagonists.J Auton Pharmacol. 1996 Dec;16(6):341-4. doi: 10.1111/j.1474-8673.1996.tb00049.x. J Auton Pharmacol. 1996. PMID: 9131412 Review.
-
Purinergic signalling: from discovery to current developments.Exp Physiol. 2014 Jan;99(1):16-34. doi: 10.1113/expphysiol.2013.071951. Epub 2013 Sep 27. Exp Physiol. 2014. PMID: 24078669 Free PMC article. Review.
Cited by
-
Neurotransmitters responsible for purinergic motor neurotransmission and regulation of GI motility.Auton Neurosci. 2021 Sep;234:102829. doi: 10.1016/j.autneu.2021.102829. Epub 2021 Jun 2. Auton Neurosci. 2021. PMID: 34146957 Free PMC article.
-
Structure activity relationship of synaptic and junctional neurotransmission.Auton Neurosci. 2013 Jun;176(1-2):11-31. doi: 10.1016/j.autneu.2013.02.012. Epub 2013 Mar 25. Auton Neurosci. 2013. PMID: 23535140 Free PMC article. Review.
-
Regional differences in nitrergic innervation of the smooth muscle of murine lower oesophageal sphincter.Br J Pharmacol. 2008 Feb;153(3):517-27. doi: 10.1038/sj.bjp.0707573. Epub 2007 Nov 26. Br J Pharmacol. 2008. PMID: 18037919 Free PMC article.
-
Evidence that ATP or a related purine is an excitatory neurotransmitter in the longitudinal muscle of mouse distal colon.Br J Pharmacol. 2007 May;151(1):73-81. doi: 10.1038/sj.bjp.0707188. Epub 2007 Mar 12. Br J Pharmacol. 2007. PMID: 17351663 Free PMC article.
-
TREK-1 channels do not mediate nitrergic neurotransmission in circular smooth muscle from the lower oesophageal sphincter.Br J Pharmacol. 2010 Jan 1;159(2):362-73. doi: 10.1111/j.1476-5381.2009.00531.x. Epub 2009 Dec 4. Br J Pharmacol. 2010. PMID: 20002101 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

