Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment
- PMID: 15677715
- PMCID: PMC546019
- DOI: 10.1073/pnas.0409736102
Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment
Abstract
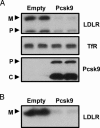
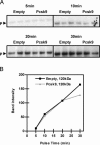
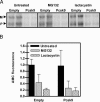
Proprotein convertase subtilisin kexin 9 (PCSK9) is a member of the subtilisin serine protease family with an important role in cholesterol metabolism. PCSK9 expression is regulated by dietary cholesterol in mice and cellular sterol levels in cell culture via the sterol regulatory element binding protein transcription factors, and mutations in PCSK9 are associated with a form of autosomal dominant hypercholesterolemia. Overexpression of PCSK9 in mice leads to increased total and low-density lipoprotein (LDL) cholesterol levels because of a decrease in hepatic LDL receptor (LDLR) protein with normal mRNA levels. To study the mechanism, PCSK9 was overexpressed in human hepatoma cells, HepG2, by adenovirus. Overexpression of PCSK9 in HepG2 cells caused a decrease in whole-cell and cell-surface LDLR levels. PCSK9 overexpression had no effect on LDLR synthesis but caused a dramatic increase in the degradation of the mature LDLR and a lesser increase in the degradation of the precursor LDLR. In contrast, overexpression of a catalytically inactive mutant PCSK9 prevented the degradation of the mature LDLR; whereas increased degradation of the precursor LDLR still occurred. The PCSK9-induced degradation of the LDLR was not affected by inhibitors of the proteasome, lysosomal cysteine proteases, aspartic acid proteases, or metalloproteases. The PCSK9-induced degradation of the LDLR was shown to require transport out of the endoplasmic reticulum. These results indicate that overexpression of PCSK9 induces the degradation of the LDLR by a nonproteasomal mechanism in a post-endoplasmic reticulum compartment.
Figures


References
-
- Bergeron, F., Leduc, R. & Day, R. (2000) J. Mol. Endocrinol. 24, 1–22. - PubMed
-
- Gensberg, K., Jan, S. & Matthews, G. M. (1998) Semin. Cell Dev. Biol. 9, 11–17. - PubMed
-
- Seidah, N. G. & Chretien, M. (1999) Brain Res. 848, 45–62. - PubMed
-
- Taylor, N. A., Van De Ven, W. J. & Creemers, J. W. (2003) FASEB J. 17, 1215–1227. - PubMed
-
- Zhou, A., Webb, G., Zhu, X. & Steiner, D. F. (1999) J. Biol. Chem. 274, 20745–20748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

