Effects of statins on vascular function of endothelin-1
- PMID: 15678081
- PMCID: PMC1576052
- DOI: 10.1038/sj.bjp.0706114
Effects of statins on vascular function of endothelin-1
Abstract
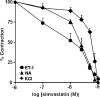
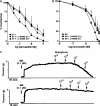
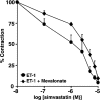
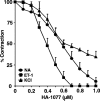
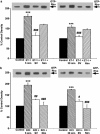
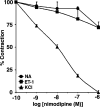
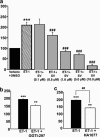
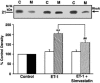
1. Although statins have been reported to inhibit the prepro-endothelin-1 (ET-1) gene transcription in endothelial cells, their effects on the vascular function of ET-1 have not been explored. We, therefore, examined the effects of statins on contraction and DNA synthesis mediated by ET-1 in vascular smooth muscle. The effects of statins on contraction induced by ET-1 were compared to those mediated by noradrenaline (NA) and KCl. 2. Simvastatin (SV) induced a concentration-dependent relaxation of tonic contraction mediated by ET-1 (10 nM) (IC50 value of 1.3 microM). The relaxation was also observed in rings precontracted with NA (0.1 microM) and KCl (60 mM). In contrast, pravastatin did not have any effect on the contractions. 3. Endothelial denudation or pretreatment with L-NAME did not prevent the relaxation, but did reduce the relaxant activity of SV. 4. SV prevented Rho activation caused by ET-1 and KCl in aortic homogenates, as assessed by a Rho pulldown assay. 5. The Rho kinase inhibitor HA-1077 mimicked the effects of SV on tonic contractions induced by ET-1, NA and KCl. 6. Pretreatment with the Kv channels inhibitor, 4-aminopyridine, attenuated the ability of SV to relax contractions mediated by ET-1 and NA. 7. In quiescent VSM cells, SV significantly inhibited DNA synthesis and Rho translocation stimulated by ET-1, as assessed by [3H]thymidine incorporation and Western blot, respectively. 8. Inhibition of Rho geranylgeranylation by GGTI-297, or treatment with HA-1077, mimicked the effects of SV on DNA synthesis stimulated by ET-1. 9. The results show that the statin potently inhibits both ET-1-mediated contraction and DNA synthesis via multiple mechanisms. Clinical benefits of statins may result, in part, from their effects on vascular function of ET-1.
Figures

Similar articles
-
Inhibition of smooth muscle cell calcium mobilization and aortic ring contraction by lactone vastatins.J Hypertens. 1996 Jan;14(1):115-21. J Hypertens. 1996. PMID: 12013483
-
Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle.Biochem J. 2006 Mar 15;394(Pt 3):581-92. doi: 10.1042/BJ20051471. Biochem J. 2006. PMID: 16336212 Free PMC article.
-
Effects of HMG-CoA reductase inhibition by simvastatin on vascular dysfunction induced by lipopolysaccharide in rats.Pharmacology. 2008;82(2):89-96. doi: 10.1159/000135629. Epub 2008 May 29. Pharmacology. 2008. PMID: 18509252
-
Elastase-induced suppression of endothelin-mediated Ca2+ entry mechanisms of vascular contraction.Hypertension. 2003 Oct;42(4):818-24. doi: 10.1161/01.HYP.0000086200.93184.8E. Epub 2003 Aug 4. Hypertension. 2003. PMID: 12900430
-
Comparison of the efficacies of five different statins on inhibition of human saphenous vein smooth muscle cell proliferation and invasion.J Cardiovasc Pharmacol. 2007 Oct;50(4):458-61. doi: 10.1097/FJC.0b013e318123767f. J Cardiovasc Pharmacol. 2007. PMID: 18049315
Cited by
-
Ggps1 deficiency in the uterus results in dystocia by disrupting uterine contraction.J Mol Cell Biol. 2021 May 7;13(2):116-127. doi: 10.1093/jmcb/mjaa066. J Mol Cell Biol. 2021. PMID: 33340314 Free PMC article.
-
Depression predicts elevated endothelin-1 in patients with coronary artery disease.Psychosom Med. 2011 Jan;73(1):2-6. doi: 10.1097/PSY.0b013e3181fdfb25. Epub 2010 Oct 14. Psychosom Med. 2011. PMID: 20947777 Free PMC article.
-
Emerging antenatal therapies for congenital diaphragmatic hernia-induced pulmonary hypertension in preclinical models.Pediatr Res. 2021 May;89(7):1641-1649. doi: 10.1038/s41390-020-01191-x. Epub 2020 Oct 10. Pediatr Res. 2021. PMID: 33038872 Free PMC article. Review.
-
Severe depressive symptoms are associated with elevated endothelin-1 in younger patients with acute coronary syndrome.J Psychosom Res. 2014 Nov;77(5):430-4. doi: 10.1016/j.jpsychores.2014.07.019. Epub 2014 Aug 2. J Psychosom Res. 2014. PMID: 25129849 Free PMC article.
-
Molecular programming of endothelin-1 in HIV-infected brain: role of Tat in up-regulation of ET-1 and its inhibition by statins.FASEB J. 2007 Mar;21(3):777-89. doi: 10.1096/fj.06-7054com. Epub 2006 Dec 28. FASEB J. 2007. PMID: 17197385 Free PMC article.
References
-
- BERG T. Analysis of the pressor response to the K+ channel inhibitor 4- aminopyridine. Eur. J. Pharmacol. 2002;452:325–337. - PubMed
-
- BERGDAHL A., PERSSON E., HELLSTRAND P., SWÄRD K. Lovastatin induces relaxation and inhibits L-type Ca2+ current in the rat basilar artery. Pharmacol. Toxicol. 2003;93:128–134. - PubMed
-
- BOBIK A., GROOMS A., MILLAR J.A., MITCHELL A., GRINPUKEL S. Growth factor activity of endothelin in vascular smooth muscle. Am J Physiol. 1990;258:C408–C415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

