Role of descending noradrenergic system and spinal alpha2-adrenergic receptors in the effects of gabapentin on thermal and mechanical nociception after partial nerve injury in the mouse
- PMID: 15678083
- PMCID: PMC1576051
- DOI: 10.1038/sj.bjp.0706109
Role of descending noradrenergic system and spinal alpha2-adrenergic receptors in the effects of gabapentin on thermal and mechanical nociception after partial nerve injury in the mouse
Abstract
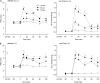
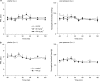
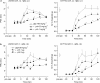
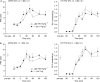
1. To gain further insight into the mechanisms underlying the antihyperalgesic and antiallodynic actions of gabapentin, a chronic pain model was prepared by partially ligating the sciatic nerve in mice. The mice then received systemic or local injections of gabapentin combined with either central noradrenaline (NA) depletion by 6-hydroxydopamine (6-OHDA) or alpha-adrenergic receptor blockade. 2. Intraperitoneally (i.p.) administered gabapentin produced antihyperalgesic and antiallodynic effects that were manifested by elevation of the withdrawal threshold to a thermal (plantar test) or mechanical (von Frey test) stimulus, respectively. 3. Similar effects were obtained in both the plantar and von Frey tests when gabapentin was injected intracerebroventricularly (i.c.v.) or intrathecally (i.t.), suggesting that it acts at both supraspinal and spinal loci. This novel supraspinal analgesic action of gabapentin was only obtained in ligated neuropathic mice, and gabapentin (i.p. and i.c.v.) did not affect acute thermal and mechanical nociception. 4. In mice in which central NA levels were depleted by 6-OHDA, the antihyperalgesic and antiallodynic effects of i.p. and i.c.v. gabapentin were strongly suppressed. 5. The antihyperalgesic and antiallodynic effects of systemic gabapentin were reduced by both systemic and i.t. administration of yohimbine, an alpha2-adrenergic receptor antagonist. By contrast, prazosin (i.p. or i.t.), an alpha1-adrenergic receptor antagonist, did not alter the effects of gabapentin. 6. It was concluded that the antihyperalgesic and antiallodynic effects of gabapentin are mediated substantially by the descending noradrenergic system, resulting in the activation of spinal alpha2-adrenergic receptors.
Figures
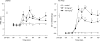
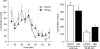
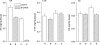


References
-
- ARNER S., MEYERSON B.A. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11–23. - PubMed
-
- BACKONJA M., BEYDOUN A., EDWARDS K.R., SCHWARTZ S.L., FONSECA V., HES M., LAMOREAUX L., GAROFALO E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. J. Am. Med. Assoc. 1998;280:1831–1836. - PubMed
-
- BAYER K., AHMADI S., ZEILHOFER H.U. Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology. 2004;46:743–749. - PubMed
-
- BERTRAND S., NG G.Y.K., PURISAI M.G., WOLFE S.E., SEVERIDT M.W., NOUEL D., ROBITAILLE R., LOW M.J., O'NEILL G.P., METTERS K., LACAILLE J.C., CHRONWALL B.M., MORRIS S.J. The anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain γ-aminobutyric acid type B receptors negatively coupled to voltage-dependent calcium channels. J. Pharmacol. Exp. Ther. 2001;298:15–24. - PubMed
-
- CHAPLAN S.R., BACH F.W., POGREL J.W., CHUNG J.M., YAKSH T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

