Addition of a signal peptide sequence to the alpha1D-adrenoceptor gene increases the density of receptors, as determined by [3H]-prazosin binding in the membranes
- PMID: 15678090
- PMCID: PMC1576044
- DOI: 10.1038/sj.bjp.0706087
Addition of a signal peptide sequence to the alpha1D-adrenoceptor gene increases the density of receptors, as determined by [3H]-prazosin binding in the membranes
Abstract
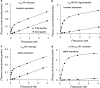
1. Both in mammalian tissues and in transfected cells, only low levels of alpha1D-adrenoceptors are detected in radioligand binding studies. It has been implicated that the comparatively long N-terminal tail of the alpha1D-adrenoceptor is responsible for the inefficient surface expression of the receptor. 2. In the present study, we created gene constructs for six N-terminally truncated variants of the human alpha1D-adrenoceptor. These constructs were used to transfect Neuro2A cells. We show that the density of alpha1D-adrenoceptors, observed by [3H]-prazosin binding, gradually increased with longer truncations of the N-terminus. This seems to indicate that the long N-terminal tail nonspecifically interferes with receptor translocation to the plasma membrane. 3. The addition of a 16 amino acids long signal peptide to the N-terminus of the wild-type alpha1D-adrenoceptor increased the density of receptor binding sites 10-fold in Neuro2A and COS-7 cells. This indicates that, after the addition of a signal peptide, the long N-terminal tail of the alpha1D-adrenoceptor does not interfere with proper translocation of the receptor to the plasma membrane. This, in turn, indicates that the N-terminal tail of the wild-type alpha1D-adrenoceptor, merely by its long length, hinders the first transmembrane helix of the receptor from being a signal anchor. 4. Neither the wild-type alpha1D-adrenoceptor (for which the expression level of [3H]-prazosin binding sites is low) nor the truncated alpha1D-adrenoceptor variant (for which the expression level of [3H]-prazosin binding sites is high) showed any constitutive activity in stimulating inositol phosphate accumulation. This indicates that the low expression level of [3H]-prazosin binding sites, after transfection with the wild-type alpha1D-adrenoceptor, is not caused by constitutive activity of the receptor and subsequent receptor downregulation.
Figures


Similar articles
-
Amino acids of the human alpha1d-adrenergic receptor involved in antagonist binding.J Pharmacol Sci. 2008 Jan;106(1):114-20. doi: 10.1254/jphs.fp0071412. Epub 2008 Jan 11. J Pharmacol Sci. 2008. PMID: 18187928
-
The predicted N-terminal signal sequence of the human α₂C-adrenoceptor does not act as a functional cleavable signal peptide.Eur J Pharmacol. 2012 Jun 5;684(1-3):51-8. doi: 10.1016/j.ejphar.2012.03.044. Epub 2012 Apr 3. Eur J Pharmacol. 2012. PMID: 22503931
-
Alpha1-adrenoceptors in the rat cerebral cortex: new insights into the characterization of alpha1L- and alpha1D-adrenoceptors.Eur J Pharmacol. 2010 Sep 1;641(1):41-8. doi: 10.1016/j.ejphar.2010.05.016. Epub 2010 May 23. Eur J Pharmacol. 2010. PMID: 20511116
-
Truncated isoforms inhibit [3H]prazosin binding and cellular trafficking of native human alpha1A-adrenoceptors.Biochem J. 1999 Oct 1;343 Pt 1(Pt 1):231-9. Biochem J. 1999. PMID: 10493934 Free PMC article.
-
Internalization and distribution of three alpha1-adrenoceptor subtypes in HEK293A cells before and after agonist stimulation.Acta Pharmacol Sin. 2007 Mar;28(3):359-66. doi: 10.1111/j.1745-7254.2007.00509.x. Acta Pharmacol Sin. 2007. PMID: 17302998
Cited by
-
Scribble co-operatively binds multiple α1D-adrenergic receptor C-terminal PDZ ligands.Sci Rep. 2019 Oct 1;9(1):14073. doi: 10.1038/s41598-019-50671-6. Sci Rep. 2019. PMID: 31575922 Free PMC article.
-
Dynamic mass redistribution reveals diverging importance of PDZ-ligands for G protein-coupled receptor pharmacodynamics.Pharmacol Res. 2016 Mar;105:13-21. doi: 10.1016/j.phrs.2016.01.003. Epub 2016 Jan 7. Pharmacol Res. 2016. PMID: 26773201 Free PMC article.
-
N-glycosylation of α1D-adrenergic receptor N-terminal domain is required for correct trafficking, function, and biogenesis.Sci Rep. 2020 Apr 29;10(1):7209. doi: 10.1038/s41598-020-64102-4. Sci Rep. 2020. PMID: 32350295 Free PMC article.
-
The C-terminal half of the α2C-adrenoceptor determines the receptor's membrane expression level and drug selectivity.Naunyn Schmiedebergs Arch Pharmacol. 2013 Dec;386(12):1031-40. doi: 10.1007/s00210-013-0902-z. Epub 2013 Jul 20. Naunyn Schmiedebergs Arch Pharmacol. 2013. PMID: 23868076
-
Rescue of misrouted GnRHR mutants reveals its constitutive activity.Mol Endocrinol. 2012 Jul;26(7):1179-88. doi: 10.1210/me.2012-1089. Epub 2012 May 17. Mol Endocrinol. 2012. PMID: 22595961 Free PMC article.
References
-
- ANDERSSON H., D'ANTONA A.M., KENDALL D.A., VON HEIJNE G., CHIN C.N. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Mol. Pharmacol. 2003;64:570–577. - PubMed
-
- CHALOTHORN D., MCCUNE D.F., EDELMANN S.E., GARCIA-CAZARIN M.L., TSUJIMOTO G., PIASCIK M.T. Differences in the cellular localization and agonist-mediated internalization properties of the α1-adrenoceptor subtypes. Mol. Pharmacol. 2002;6:1008–1016. - PubMed
-
- GARCIA-SAINZ J.A., TORRES-PADILLA M.E. Modulation of basal intracellular calcium by inverse agonists and phorbol myristate acetate in rat-1 fibroblasts stably expressing α1d-adrenoceptors. FEBS Lett. 1999;443:277–281. - PubMed
-
- GARCIA-SAINZ J.A., VILLALOBOS-MOLINA R. The elusive α1D-adrenoceptor: molecular and cellular characteristics and integrative roles. Eur. J. Pharmacol. 2004;500:113–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

